��Ŀ����
����Ŀ���״���һ����Ҫ���л�����ԭ�ϡ�
��1����֪��
��C2H4(g)+H2O(g)��C2H5OH(g) ��H1=-45.5 kJ/mol
��2CH3OH(g)��CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol
��C2H5OH(g)��CH3OCH3(g) ��H3=+50.7 kJ/mol
��д����ϩ��ˮ�����������ɼ״�������Ȼ�ѧ����ʽ��__________��
��2���ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
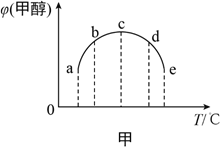
���¶����״���������������ԭ����__________��
�ڸ���ͼ���жϦ�H__________���>������<����=����0��
��3��Ϊ���о��״�ת��Ϊ�����ѵķ�Ӧ������ij�о���С�������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g) ![]() CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | T1 | 0.20 | 0.080 | 0.080 |
�� | T1 | 0.40 | A | a |
�� | T2 | 0.20 | 0.090 | 0.090 |
��T1�¶��¸÷�Ӧ��ƽ�ⳣ��K=__________����Ӧ�¶�T1__________T2������ڡ���С�ڡ�����
����������a=__________��
������˵����˵����Ӧ�ﵽƽ��״̬����__________������ĸ����
A������������ѹǿ���ٱ仯
B����CH3OH��CH3OCH3��ʾ�ķ�Ӧ����֮��Ϊ2:1
C�����������ܶȲ���
D��������CH3OH��CH3OCH3��Ũ��֮��Ϊ2:1
E�����������c(CH3OCH3)����
���𰸡� C2H4(g)+2H2O(g)��2CH3OH(g) ��H=+29.1 kJ/mol a��c��Ӧδ��ƽ�⣬�¶�Խ�߷�Ӧ����Խ�죬�״����������Խ�� < 4 ���� 0.16 E
����������1�����ݸ�˹���ɢ�-��+�ۿɵ�C2H4(g)+2H2O(g) ![]() 2CH3OH(g)����H=��H1-��H2+��H3=+29.1 kJ/mol���ʴ�Ϊ��C2H4(g)+2H2O(g)
2CH3OH(g)����H=��H1-��H2+��H3=+29.1 kJ/mol���ʴ�Ϊ��C2H4(g)+2H2O(g) ![]() 2CH3OH(g) ��H=+29.1kJ/mol��
2CH3OH(g) ��H=+29.1kJ/mol��
��2����a��b��û�дﵽƽ����c��ǡ��ƽ�⣬d��eΪƽ��״̬��a��c֮��ĵ������ƽ��״̬���¶����ߣ���Ӧ���ʼӿ죬��ͬʱ���ڣ����ɵļ״����࣬�״��������������
�ʴ�Ϊ��a��c��Ӧδ��ƽ�⣬�¶�Խ�߷�Ӧ����Խ�죬�״����������Խ��
��c��e֮��ĵ��Ϊƽ��״̬���¶����ߣ�ƽ�������ȷ����ƶ����÷�Ӧ���¶��������״������������С����ƽ�����淴Ӧ�����ƶ�����������ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
��3�������ݱ������ݣ��г�����I�з�Ӧ����ʽ��
2CH3OH(g) ![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��ʼ���ʵ���/mol 0.20 0 0
�仯���ʵ���/mol 0.16 0.080 0.080
ƽ�����ʵ���/mol 0.04 0.080 0.080
�������Ϊ1L����ƽ�ⳣ��K=![]() =
=![]() =4��
=4��
�÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���CH3OCH3(g)������С������I������������ʼʱͶ���CH3OH���ʵ�����ͬ����ƽ��ʱ����CH3OCH3(g)�����ͣ�˵��������ķ�Ӧ�¶Ƚϸ�����T1����T2��
�ʴ�Ϊ��4�����ڣ�
����Ӧ2CH3OH(g) ![]() CH3OCH3(g)+H2O(g)�Ƿ�Ӧǰ�������������ķ�Ӧ�����º���������
CH3OCH3(g)+H2O(g)�Ƿ�Ӧǰ�������������ķ�Ӧ�����º���������
���������ڵ�Чƽ���������ʼ���ʵ�����I��2������ƽ��ʱ����������ҲΪ���2������a=0.08��2=0.16��
�ʴ�Ϊ��0.16��
�۵��������ٸı�ʱ����Ӧ�ﵽƽ��״̬��A���÷�ӦΪ��Ӧǰ��������Ŀ���䣬�����������ʵ���n�Ƕ���������pV=nRT��֪�����º���ʱ��p��n�����ȣ�����������ѹǿΪ��������ѹǿ���ٱ仯����˵����Ӧ�ﵽƽ��״̬����A����B������Ӧ�У�CH3OH��CH3OCH3�ķ�Ӧ����֮��һֱΪ2:1���淴Ӧ�У�CH3OH��CH3OCH3�ķ�Ӧ����֮��ҲһֱΪ2:1������CH3OH��CH3OCH3������֮��Ϊ2:1����˵����Ӧ�ﵽƽ��״̬����B����C����=m/V��������������Ƕ���������ʱ���Ҳ�Ƕ��������ܶ�Ϊ�������ܶȲ��䲻��˵����Ӧ�ﵽƽ��״̬����C����D��ƽ��ʱCH3OH��CH3OCH3��Ũ��֮�ȿ���Ϊ2:1��Ҳ���ܲ���2:1������˵����Ӧ�ﵽƽ��״̬����D����E��c(CH3OCH3)�DZ�������c(CH3OCH3)���䣬˵����Ӧ�ﵽƽ��״̬����E��ȷ��
�ʴ�Ϊ��E��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��2N2O5(g) ![]() 4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
t/s | 0 | 500 | 1000 | 1500 |
c(N2O5)/(mol��L-1) | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ���ǣ� ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10-3 mol��L-1��s-1
B. �����������䣬T2�¶��·�Ӧ��1000sʱ���N2O5(g)Ũ��Ϊ2.98 mol��L-1����T1<T2
C. T1�¶��µ�ƽ�ⳣ��ΪK1=125��1000sʱת����Ϊ50%
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1>K3����T1>T3