��Ŀ����
����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��2N2O5(g) ![]() 4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
t/s | 0 | 500 | 1000 | 1500 |
c(N2O5)/(mol��L-1) | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ���ǣ� ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10-3 mol��L-1��s-1
B. �����������䣬T2�¶��·�Ӧ��1000sʱ���N2O5(g)Ũ��Ϊ2.98 mol��L-1����T1<T2
C. T1�¶��µ�ƽ�ⳣ��ΪK1=125��1000sʱת����Ϊ50%
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1>K3����T1>T3
���𰸡�B
��������A������ͼ�����ݷ�������500s����c(N2O5)=(5.00-3.52)mol/L=1.48mol/L��v(N2O5)= ![]() =2.96��10-3mol/��Ls������A��ȷ��B������T1<T2������ӦΪ���ȷ�Ӧ�������������䣬�����¶ȣ���Ӧ���ʼӿ죬�ﵽƽ���ʱ�����̣���1000sʱ����Ӧ���Ѵﵽƽ�⣬�����¶���ƽ�������ƶ�����ƽ��ʱN2O5��Ũ��ӦС��2.50mol/L��T1<T2����������B����C���ɱ������ݿ�֪��T1�¶��£�1000sʱ��Ӧ����ƽ�⣬ƽ��ʱc(N2O5)=2.5mol/L��c(NO2)=5mol/L��c(O2)=1.25mol/L��ƽ�ⳣ��K=
=2.96��10-3mol/��Ls������A��ȷ��B������T1<T2������ӦΪ���ȷ�Ӧ�������������䣬�����¶ȣ���Ӧ���ʼӿ죬�ﵽƽ���ʱ�����̣���1000sʱ����Ӧ���Ѵﵽƽ�⣬�����¶���ƽ�������ƶ�����ƽ��ʱN2O5��Ũ��ӦС��2.50mol/L��T1<T2����������B����C���ɱ������ݿ�֪��T1�¶��£�1000sʱ��Ӧ����ƽ�⣬ƽ��ʱc(N2O5)=2.5mol/L��c(NO2)=5mol/L��c(O2)=1.25mol/L��ƽ�ⳣ��K= =125��ת����=
=125��ת����=![]() =50%����C��ȷ��D��ƽ�ⳣ��ֻ���¶�Ӱ�죬T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1��K3����Ӧ���ȷ�Ӧ����T1��T3����D��ȷ����ѡB��
=50%����C��ȷ��D��ƽ�ⳣ��ֻ���¶�Ӱ�죬T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1��K3����Ӧ���ȷ�Ӧ����T1��T3����D��ȷ����ѡB��

����Ŀ���״���һ����Ҫ���л�����ԭ�ϡ�
��1����֪��
��C2H4(g)+H2O(g)��C2H5OH(g) ��H1=-45.5 kJ/mol
��2CH3OH(g)��CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol
��C2H5OH(g)��CH3OCH3(g) ��H3=+50.7 kJ/mol
��д����ϩ��ˮ�����������ɼ״�������Ȼ�ѧ����ʽ��__________��
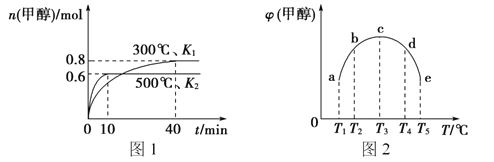
��2���ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
���¶����״���������������ԭ����__________��
�ڸ���ͼ���жϦ�H__________���>������<����=����0��
��3��Ϊ���о��״�ת��Ϊ�����ѵķ�Ӧ������ij�о���С�������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g) ![]() CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | T1 | 0.20 | 0.080 | 0.080 |
�� | T1 | 0.40 | A | a |
�� | T2 | 0.20 | 0.090 | 0.090 |
��T1�¶��¸÷�Ӧ��ƽ�ⳣ��K=__________����Ӧ�¶�T1__________T2������ڡ���С�ڡ�����
����������a=__________��
������˵����˵����Ӧ�ﵽƽ��״̬����__________������ĸ����
A������������ѹǿ���ٱ仯
B����CH3OH��CH3OCH3��ʾ�ķ�Ӧ����֮��Ϊ2:1
C�����������ܶȲ���
D��������CH3OH��CH3OCH3��Ũ��֮��Ϊ2:1
E�����������c(CH3OCH3)����