��Ŀ����
����Ŀ������������(V2O5,Ħ������Ϊ182g��mol-1)������ѧ��ҵ�еĴ������㷺����ұ�𡢻�������ҵ��V2O5��һ�ֳȻ�ɫƬ״����,����ˮ���������Ҵ�,����ǿ������,�������������ij�о�С�齫��ij�ַ�(��Ҫ����V2O5,��������Al2O3��Fe2O3)����ȡV2O5��ʵ�鷽��������£�

��֪��NH4VO3�ǰ�ɫ��ĩ,������ˮ,��������ˮ,�������Ҵ����ѡ�
2NH4VO3![]() V2O5+2NH3��+H2O
V2O5+2NH3��+H2O
��ش�
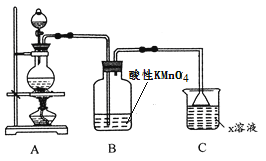
��1��������������ʵ��װ������ͼ��ʾ,���߿�����Ϊ���ʵ�������________��(����)
![]()
![]()
![]()
![]()

��2������pHΪ8��8.5��Ŀ��________��
��3��������ϴ�Ӳ���ʱ,��ѡ�õ�ϴ�Ӽ�_________��(����)
A����ˮ B����ˮ C���Ҵ� D��1%NH4Cl��Һ
��4������������ʱ,�����������������յĿ���ԭ��________��
��5�����Ṥҵ��,SO2ת��ΪSO3�Ĵ�����ѡ��V2O5,�����̾��������,���䲹��������________(�û�ѧ����ʽ��ʾ),4VO2+O2=2V2O5��
��6����0.253g��Ʒ����ǿ����Һ��,�������,����pHΪ8��8.5,��Ӧ�����Һ�м��������ữ��KI��Һ(����),��Һ�к���V3+,�μ�ָʾ��,��0.250mol��L-1Na2S2O3��Һ�ζ�,�ﵽ�յ�����Na2S2O3����Һ20.00mL,��ò�Ʒ�Ĵ���Ϊ________��(��֪��I2+2Na2S2O3=Na2S4O6+2NaI)
���𰸡� B ��AlO2-ת��ΪAl(OH)3����������VO3-�ij��� A D ����������ͨ������V2O5����ǿ�����ԣ����뻹ԭ�Ե�NH3��Ӧ���Ӷ�Ӱ�����Ĵ��ȼ����� SO2+V2O5=2VO2+SO3 89.9%
�����������⿼��ʵ�鷽����������ۣ���1�����߿��е���������������ƿ�еμ�NaOH��Ϊ���ܹ�ʹNaOH��Һ˳�����£���Ҫ����ͨװ�ã���ѡ��B��ȷ����2����������ͼ��������Ϊ�������������NaOH������Ӧ��V2O5��Al2O3�����������������NaOH������Ӧ������pHΪ8��8.5��Ŀ�ľ�����AlO2��ת��ΪAl(OH)3����������VO3���ij�������3��������Ϣ��NH4VO3����ˮ����������ˮ���������Ҵ����ѣ�A������ˮϴ�ӣ����Լ���NH4VO3���ܽ⣬��A��ȷ��B��NH4VO3������ˮ�����NH4VO3�ܽ⣬��B����C����ȻNH4VO3�������Ҵ�����NH4VO3�������ʣ���NH4Cl�������Ҵ���ʹ���Ҵ�����ϴȥ������������ʣ���C����D��������������NH4VO3����������Һ1�뱥��NH4Cl�ķ�Ӧ���ɣ���NH4Cl�����ֽ⣬���������ʣ���D��ȷ����4��������Ϣ��NH4VO4��������V2O5����ΪV2O5����ǿ�����ԣ�������л�ԭ��NH3������Ӧ���Ӷ�Ӱ�����Ĵ��ȼ����ʣ��������NH4VO3ʱ�����������У���5�������ڷ�Ӧǰ���������䣬������֪��Ӧ��Ӧ����ʽ����˵ó���Ӧ����ʽΪSO2��V2O5=2VO2��SO3����6������I2��Na2S2O3������Ӧ�ķ���ʽ���������n(I2)=20.00��10��3��0.250/2mol=2.5��10��3mol�����ݵ�ʧ������Ŀ�غ㣬n(V2O5)��2��2=n(I2)��2�����n(V2O5)=1.25��10��3mol����m(V2O5)=1.25��10��3��182g=0.2275g�����Ʒ����Ϊ0.2275/0.253��100%=89.9%��

����Ŀ���״���һ����Ҫ���л�����ԭ�ϡ�
��1����֪��
��C2H4(g)+H2O(g)��C2H5OH(g) ��H1=-45.5 kJ/mol
��2CH3OH(g)��CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol
��C2H5OH(g)��CH3OCH3(g) ��H3=+50.7 kJ/mol
��д����ϩ��ˮ�����������ɼ״�������Ȼ�ѧ����ʽ��__________��
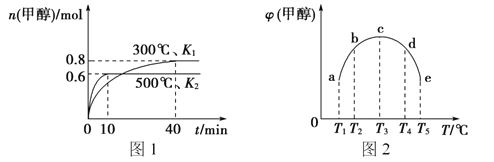
��2���ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
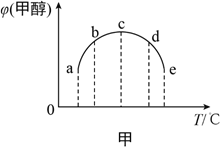
���¶����״���������������ԭ����__________��
�ڸ���ͼ���жϦ�H__________���>������<����=����0��
��3��Ϊ���о��״�ת��Ϊ�����ѵķ�Ӧ������ij�о���С�������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g) ![]() CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | T1 | 0.20 | 0.080 | 0.080 |
�� | T1 | 0.40 | A | a |
�� | T2 | 0.20 | 0.090 | 0.090 |
��T1�¶��¸÷�Ӧ��ƽ�ⳣ��K=__________����Ӧ�¶�T1__________T2������ڡ���С�ڡ�����
����������a=__________��
������˵����˵����Ӧ�ﵽƽ��״̬����__________������ĸ����
A������������ѹǿ���ٱ仯
B����CH3OH��CH3OCH3��ʾ�ķ�Ӧ����֮��Ϊ2:1
C�����������ܶȲ���
D��������CH3OH��CH3OCH3��Ũ��֮��Ϊ2:1
E�����������c(CH3OCH3)����