��Ŀ����
18��C��N��O��Al��Fe��Cu�dz���������Ԫ�أ���1��Feλ��Ԫ�����ڱ��ĵ������ڵڢ����壬Cu�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ3d104s1��
Alԭ�ӵĻ�̬ԭ�Ӻ�����13���˶�״̬��ͬ�ĵ��ӣ�
��2���á�����������գ�
| �縺�� ���Ӱ뾶 ���ļ��� | �۵� |
| N��O O2-��Al3+ C-H��H-O | Al��Al2O3 |
���壨NO2��NO������Һ�в���4.4g���壮д��������Ӧ���ܵĻ�ѧ����ʽ5Fe+14HNO3$\frac{\underline{\;\;��\;\;}}{\;}$5Fe��NO3��2+NO2��+3NO��+7H2O��
��4��Fe��s��+O2��g��=FeO��s����H=-272.0kJ/mol
Al��s��+O2��g��=Al2O3��s����H=-1675.7kJ/mol
Al�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ•mol-1��
���� ��1��Fe�ĺ��������Ϊ26��ԭ����4�����Ӳ㣬�۲������Ϊ8��Cu�ĺ��������Ϊ29��ԭ����4�����Ӳ㣬�۲������Ϊ11��Al�ĺ��������Ϊ13��
��2�����ݷǽ�����Խǿ���縺��Խ���жϣ�
���ݺ�������Ų���ͬʱ��ԭ������ԽС���뾶Խ��
����Ԫ�صķǽ�����Խǿ�����ļ���Խǿ��
���ݼ�����Ƭʱ�����ۻ��������·����жϣ�
��3����10g��������40mlHNO3��Һ�У���Ӧ����������Ϊ��10g-4.4g=5.6g�����ݱ���ԭ����������ʵ���Ϊ��$\frac{1.792L}{22.4L/mol}$=0.08mol��δ����ԭ��HNO3�����ʵ���Ϊn��HNO3��=2n��Fe��=2��0.1mol=0.2mol����μӷ�Ӧ������Ϊ0.2+0.08=0.28mol�����Բμӷ�Ӧ����������֮��Ϊ0.1��0.28=5��14�����ݷ�Ӧ���йط���ʽ���Ԫ���غ����Լ������غ������д��
��4�����ݢ�Fe��s��+$\frac{1}{2}$O2��g��=FeO��s����H=-272.0kJ•mol-1
��2Al��s��+$\frac{3}{2}$O2��g��=Al2O3��s����H=-1675.7kJ•mol-1
������ʽ��-�١�3��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s���ݴ˼����ʱ䣮
��� �⣺��1����ΪFe�ĺ��������Ϊ26��ԭ����4�����Ӳ㣬�۲������Ϊ8������Feλ��Ԫ�����ڱ��ĵ������ڵڢ����壻Cu�ĺ��������Ϊ29��ԭ����4�����Ӳ㣬�۲������Ϊ11������Cu�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ3d104s1��Al�ĺ��������Ϊ13��ÿһ�ֵ��ӵ��˶�״̬����ͬ������Alԭ�ӵĻ�̬ԭ�Ӻ�����13���˶�״̬��ͬ�ĵ��ӣ��ʴ�Ϊ���ģ�����3d104s1��13��
��2����Ϊ�ǽ�����Խǿ���縺��Խ������N�ĵ縺��С��O��
��Ϊ��������Ų���ͬʱ��ԭ������ԽС���뾶Խ������O2-�İ뾶����Al3+��
��ΪԪ�صķǽ�����Խǿ�����ļ���Խǿ������C-H�ļ���С��H-O��
��Ϊ������Ƭʱ�����ۻ��������£�˵������������û���ۻ��������۵�AlС��Al2O3��
�ʴ�Ϊ������������������
��3����Ӧ����������Ϊ��10g-4.4g=5.6g����n��Fe��=$\frac{5.6g}{56g/mol}$=0.1mol����ԭ����������ʵ���Ϊ��$\frac{1.792L}{22.4L/mol}$=0.08mol��δ����ԭ��HNO3�����ʵ���Ϊn��HNO3��=2n��Fe��=2��0.1mol=0.2mol����μӷ�Ӧ������Ϊ0.2+0.08=0.28mol�����Բμӷ�Ӧ����������֮��Ϊ0.1��0.28=5��14�����������������ɶ�����������Ӧ�ķ���ʽΪ��5Fe+14HNO3$\frac{\underline{\;\;��\;\;}}{\;}$5Fe��NO3��2+NO2��+3NO��+7H2O���ʴ�Ϊ��5Fe+14HNO3$\frac{\underline{\;\;��\;\;}}{\;}$5Fe��NO3��2+NO2��+3NO��+7H2O��
��4����Fe��s��+$\frac{1}{2}$O2��g��=FeO��s����H=-272.0kJ•mol-1
��2Al��s��+$\frac{3}{2}$O2��g��=Al2O3��s����H=-1675.7kJ•mol-1
������ʽ��-�١�3��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ•mol-1��
�ʴ�Ϊ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ•mol-1��
���� �����ǵ��ۺ��⣬�����֪ʶ��϶࣬�漰��ԭ�ӽṹ�����ʵıȽϡ�������ԭ��Ӧ����ʽ����д�Լ���˹���ɵ�Ӧ�ã���Ŀ�ѶȲ���ע���йط�Ӧ����ʽ����д��

| A�� | ͭ��һ���Ϻ�ɫ���� | |
| B�� | ͭ���Ⱥ͵�������� | |
| C�� | ͭ������ʹ�����硢Ӧ����㷺�Ľ���֮һ | |
| D�� | ͭ����Ȼ������Ҫ�Ե��ʵ���ʽ���� |
| A�� | c��H+��=c��OH-������Һ�У�SO42-��Na+��Cl-��Fe3+ | |
| B�� | ��c��HCO3-��=0.1mol•L-1����Һ�У�[Al��OH��4]-��Na+��NO3-��C6H5O- | |
| C�� | ������ˮ�У�K+��Mg2+��CH3COO-��SO42- | |
| D�� | ��ʹ��̪������Һ��Ba2+��NO3-��I-��Na+ |
| A�� | ��Һ��ˮ�������H+�������ڣ��� | |
| B�� | ����Һ�������ӵ���Ũ��С��0.01 mol•L-1 | |
| C�� | ����Һ�У�n��OH-��=n��HCO3-��+2n��H2CO3��+n��H+�� | |
| D�� | ����Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� |
| A�� | CH2O��CO2�����е�����ԭ�Ӿ�����sp2�ӻ� | |
| B�� | �װ�������ʽCH3NH2���ķе�����װ�������ʽ��CH3��3N���ķе�� | |
| C�� | C6H6�����к���6���Ҽ���1����м���C6H6�ǷǼ��Է��� | |
| D�� | CH3SH ����Է���������CH3OH�����ǰ�ߵķе�� |
| A�� | ���зǼ��Թ��ۼ��Ļ��������һ���ǷǼ��Է��� | |
| B�� | ԭ�Ӿ�����ֻ���ڷǼ��Թ��ۼ� | |
| C�� | ����Ҫ��ˮ����ͨ��������ö��γɵķ��Ӿ��� | |
| D�� | ��Ԫ��R�ĺ���������Դ���Ԫ��Q�ĺ���������ԣ���ǽ�����R����Q |
| A�� | CO2��ˮ��Һ������������������CO2��������� | |
| B�� | ǿ�����һ����������ˮ�Ļ�����������һ����������ˮ�Ļ����� | |
| C�� | ��ˮ��Һ�������Ϊ�����ƶ������ӵĻ������ǵ���� | |
| D�� | ��ǿ����ʵ�ˮ��Һ��ֻ������û�з��� |
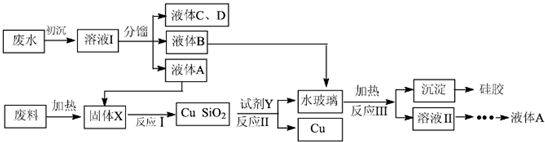
 ��
�� ��
��