��Ŀ����
����Ŀ��С���ڿ����Ķ��е�֪��������ͭ���Ȼ�ֽ���������ͭ��Ca(OH)2=CuO+X����������CuSO4��Һ��KOH��Һ��Ӧ��ȡ������ͭ������������ͭ���м��ȡ�
(1)������X�Ļ�ѧʽΪ____��
(2)��ȡ������ͭ�Ļ�ѧ����ʽΪ____��
(3)С���ڼ���������ͭʱ��������ɫ�����ȱ�ɺ�ɫ�������������պ�ɫ�����ɺ�ɫ��ͬʱ�����������ΪŪ�����ֺ�ɫ����ijɷݣ����������µ�̽����
���������ϣ�Cu��Cu2O��Ϊ��ɫ���壬��Cu2O+H2SO4=Cu+CuSO4+H2O��
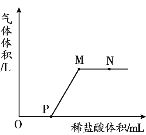
��������룩��ɫ�����ǣ���.Cu����.Cu2O����.��____��
���� | ���� | ���� |
��ȡ���պ�ĺ�ɫ����1.44g���Թ��У���������ϡ���ᣬ���Ȳ���������á� | ��Һ����ɫ����ɫ��������١� | 1.44g��ɫ����һ���У���____�� |
�ڹ��ˡ�ϴ�ӡ���� | �ú�ɫ���� |
������ʵ�飩
�����۷�����
�۾�����ʵ������С����Ϊ�������ȷ������ͬѧ��ΪС�����жϲ��Ͻ�����Ϊ����___Ҳ����ͬ������
��Ϊ�˽�һ��ȷ��1.44g��ɫ����ijɷ֣�ͬѧ�ǽ���ַ�Ӧ�Ĺ�����ˡ�ϴ�ӡ���������Ϊ1.24g��ͨ�������ȷ���������ȷ����������1.44g��ɫ������CuΪ____g��Cu2OΪ______g��
���𰸡�H2O CuSO4+2KOH=Cu��OH��2��+K2SO4 Cu��Cu2O Cu2O �� 1.08 0.36
��������
�⣺(1)���ݷ�Ӧ�Ļ�ѧ����ʽCu��OH��2�TCuO+X����Ӧ����ͭ���⡢��ԭ�Ӹ����ֱ�Ϊ1��2��2����Ӧ�����������ͭ���⡢��ԭ�Ӹ����ֱ�Ϊ1��0��1�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��X�к���2����ԭ�Ӻ�1����ԭ�ӣ�������X�Ļ�ѧʽΪH2O��
(2)CuSO4��Һ��KOH��Һ��Ӧ��ȡ������ͭ����ѧ����ʽΪ��CuSO4+2KOH=Cu��OH��2��+K2SO4��
(3)�ٺ�ɫ����ijɷ֣�����CuҲ��Cu2O��
ͭ�������Ӧ���ɻ�ѧ����ʽCu2O+H2SO4�TCu+CuSO4+H2O֪����Һ����ɫ����ɫ��������٣����ˡ�ϴ�ӡ�����ú�ɫ���壬˵��1.44g��ɫ����һ���У�Cu2O��
����Ϊ���������������ͬ������С����Ϊ�������ȷ�жϲ��Ͻ���
�����ɫ�����к�Cu2O������Ϊx��
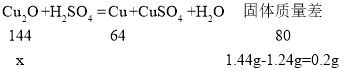
![]()
x=0.36g��
��1.44g��ɫ������Cu������=1.44g-0.36g=1.08g��

����Ŀ��С��ȡ����п�̸ɵ�ؽ���̽���������ֵ���ڲ��ṹ��ͼ��ʾ��

���������ϣ�(1)пƤΪ����п(������������)��
(2)��ɫ��״�������̿�ڡ�MnO2��ZnCl2��NH4Cl�������
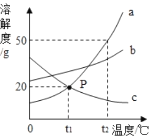
(3)�й����ݼ��±���
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | NH4Cl | 29.3 | 37.2 | 45.8 | 55.3 | 65.6 | 77.3 |
ZnCl2 | 343 | 395 | 452 | 488 | 541 | 614 | |
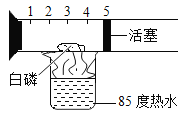
��ʵ��̽��һ��С��ȡ������ɫ��״��������ͼ��ʾʵ�����̽���̽����
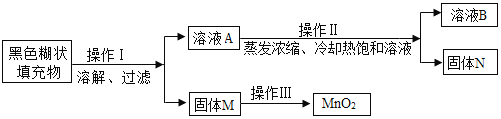
(1)��ҺA��������Ҫ��______________(�ѧʽ)���������в�����ȴ�ȱ�����Һ�ķ���ʵ�����߷����������_____________��
(2)���ӹ���M�еõ��ϴ���MnO2��������ɲ��õļ�㷽����________________________��
��ʵ��̽������С��ȡ������пƤ����ɰֽ��ĥ�ɾ�������С�飬����ʵ�顣
(1)ȡһ��пƤ����ʢ������ϡ������ձ��У���ZnCl2���ɡ�
�� ʵ���пɹ۲쵽��������____________________________���÷�Ӧ���ڻ�����Ӧ�����е�______________��Ӧ��
�� ���и������ʻ�Ϻ�Ҳ������ZnCl2����______________(����ĸ���)��
A��ZnO��ϡ���� B��ZnSO4��Һ��BaCl2��Һ
C��ZnCO3��NaCl��Һ D��Zn(NO3)2��Һ��NH4Cl��Һ
(2)��ȡһ��пƤ����ʢ��һ����CuSO4��Һ���ձ��У���ַ�Ӧ��õ���ҺE����F������ҺE��ֻ��һ������ʱ����������______________(�ѧʽ)��������F��ֻ��һ������ʱ����ҺE�����ٺ���___________�����ʡ�
(3)����6.5 gпƤ������ϡ������ȫ��Ӧ������������������______0.2 g(ѡ������������������