��Ŀ����
15�����ںͽ�������ᣬ����Դ�Ͳ��ϵ�������Խ��Խ������ѧϰ��ѧ��Ϊ����ʶ���ʣ��������ʣ���������Դ����չ�������δ�������������й���Դ�����⣺��1��δ������Դ���ص�����Դ�ḻ����ʹ��ʱ�Ի�������Ⱦ����Ⱦ��С���ҿ�����������������δ������Դ������B��
����Ȼ�� ��ú �ۺ��� ��ʯ�� ��̫���� ���������� �߷��� ������
A���٢ڢۢ�B���ݢޢߢ�C���ۢݢޢߢ�D���ۢܢݢޢߢ�
��2���˶�����ʹ�õĻ���ȼ��һ���DZ��飨C3H8������������������Ŀ��
����֪11g���飨C3H8����298K��101Kpa��������ȫȼ������CO2��Һ̬ˮʱ�ų�������Ϊ555kJ����д������ȼ���ȵ��Ȼ�ѧ����ʽ��C3H8��g��+5O2��g��=3CO2��g��+4H2O��l����H=2220 kJ•mol-1 ��
���Ա��飨C3H8���Ϳ���Ϊԭ�ϣ�ϡ����Ϊ�������Һ�ܣ����ܻ��ܣ���Ƴɳأ�
���ܣ���д��ͨ����飨C3H8������һ��Ϊ������������������������Ӧ�������ܣ��������ǣ�����
�۱�����һ�������·������ⷴӦ���Եõ���ϩ��
��֪��C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=+156.6kJ•mol-1
CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=+32.4kJ•mol-1
����ͬ�����£���ӦC3H8��g����CH3CH=CH2��g��+H2��g���ġ�H=124.2kJ•mol-1
��3����֪��H-H���ļ���Ϊ436KJ/mol��H-N���ļ���Ϊ391KJ/mol�����ݻ�ѧ����ʽ��N2+3H2?2NH3��H=-92.4KJ/mol��
��������N��N���ļ���Ϊ945.66KJ/mol��
�������������Ȼ�ѧ����ʽ��ͬ�¶Ⱥ����һ���������У�ͨ��1mol N2 ��3mol H2����ַ�Ӧ�ָ�ԭ�¶�ʱ�ų�������С�� 92.4KJ������ڻ�С�ڻ���ڣ���
���� ��1��ú��ʯ�͡���Ȼ���ǻ�ʯȼ�ϣ�̫���ܡ����ܡ����ܡ������ܡ����ܡ������ܺ��������ܵȶ�������Դ��
��2����д�ɱ���ȼ�յĻ�ѧ����ʽ��ע�����ʵľۼ�״̬������11g���飨C3H8����298K��101Kpa��������ȫȼ������CO2��Һ̬ˮʱ�ų�������Ϊ555kJ������1mol����ų��������ݴ���д��Ӧ�Ȼ�ѧ����ʽ��
���Է���������ԭ��Ӧ������Ƴ�ԭ��أ�ȼ���ڸ�������������ԭ��Ӧ������������������ԭ��Ӧ��
�۸��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ���Ժ��ʵ�ϵ�����мӼ�����Ŀ��Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ����������Ӧ�ļӼ����ݴ˼��㣻
��3���ٷ�Ӧ�С�H=���ѵĻ�ѧ���ļ���֮��-�ɼ��ļ���֮�ͣ��Դ������
�ڿ��淴Ӧ���ܽ��е��ף�
��� �⣺��1��ú��ʯ�͡���Ȼ���ǻ�ʯ��Դ�����ܲ�����������������Դ����̫���� ���������� �߷��� ��������������Դ��
��ѡ��B��
��2����11g���飨C3H8����298K��101Kpa��������ȫȼ������CO2��Һ̬ˮʱ�ų�������Ϊ555kJ������1mol����ų���������1mol����ȼ�շų�������2220 kJ����Ӧ���Ȼ�ѧ����ʽ��C3H8��g��+5O2��g��=3CO2��g��+4H2O��l����H=2220 kJ•mol-1��
�ʴ�Ϊ��C3H8��g��+5O2��g��=3CO2��g��+4H2O��l����H=2220 kJ•mol-1��
�ڱ���ȼ��Ϊ�Է���������ԭ��Ӧ��������Ƴ�ԭ��أ�ȼ���ڸ�������������ԭ��Ӧ����ͨ����飨C3H8������һ��Ϊ����������������������ԭ��Ӧ��������һ��Ϊ������
�ʴ�Ϊ���ܣ���������
����֪����C3H8��g���TCH4��g��+HC��CH��g��+H2��g����H1=156.6kJ•mol-1
��CH3CH=CH2��g���TCH4��g��+HC��CH��g ����H2=32.4kJ•mol-1
���ݸ�˹���ɣ���-�ڵ�C3H8��g���TCH3CH=CH2��g��+H2��g�������ԡ�H=��H1-��H2=156.6kJ•mol-1-32.4kJ•mol-1=124.2kJ•mol-1��
�ʴ�Ϊ��124.2kJ•mol-1��
��3������N��N���ļ�����x��N2+3H2=2NH3 ��H=-92.4KJ/mol����x+3��436KJ/mol-2��3��391KJ/mol=-92.4KJ/mol��
���x=945.6KJ/mol��
�ʴ�Ϊ��945.66KJ/mol��
��N2+3H2?2NH3��H=-92.4KJ/mol������Ϊ1mol������3mol������ȫ��Ӧ����2mol�����ų�����92.4KJ�����÷�ӦΪ���淴Ӧ��1mol������3mol����������ȫ��Ӧ�����Էų�������С��92.4KJ��
�ʴ�Ϊ��С�ڣ�
���� ����Ϊ�ۺ��⣬�漰��Դ�ķ��࣬�Ȼ�ѧ����ʽ����д�����ø�˹���ɼ��㷴Ӧ�ȣ�ȼ�ϵ�أ�Ϊ��Ƶ���㣬����ԭ��ع���ԭ������Ϥ��˹���ɼ��㷴Ӧ�ȵķ����ǽ��Ĺؼ������ط�������������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�| A�� | a�������Է�������һ����b�������Է��������� | |
| B�� | a���������CO��b���������CH4 | |
| C�� | A�����������������������B������������������� | |
| D�� | ������A���������һ������B��������� |
| A�� | ��ԭ��صĸ����͵��ص������϶�����ʧ���ӵ�������Ӧ | |
| B�� | �ö��Ե缫���Na2SO4��Һ������������������ʵ���֮��Ϊ1��2 | |
| C�� | �ö��Ե缫��ⱥ��NaCl��Һ������0.1 mol����ת�ƣ�������0.1 mol NaOH | |
| D�� | �Ʋ������п����ȶ����������ʴ |
| �ɷ� | ������g�� | Ħ��������g•mol-1�� |
| ���ǣ�C12H22O11�� | 25.00 | 342 |
| ����� | 0.84 | 174 |
| ��˾ƥ�� | 0.17 | 180 |
| ������� | 0.316 | 158 |
| ������ | 0.075 | 170 |
��2������Һ���ƹ����У���^�в��������ƽ��û��Ӱ�����BD��
A������ʱ��������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
��3��һ�ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ0.0233mol/L��

��������������������pH��������ʵ��ܽ������±���
| ��1 | ��2 | |||
| ���� | ��ʼ������pH | ������ȫ��pH | ���� | 20��ʱ�ܽ��ԣ�H2O�� |
| Fe3+ | 1.1 | 3.2 | CaSO4 | �� |
| Fe2+ | 5.8 | ����8.8 | NiF | ���� |
| Al3+ | 3.0 | 5.0 | CaF2 | ���� |
| Ni2+ | 6.7 | 9.5 | NiCO3 | KSP=9.60��10-6 |
��2������1������3��Ҫ�ɷֵĻ�ѧʽ�ֱ���SiO2��CaSO4��CaF2��
��3��д�����������м���H2O2������Ӧ�����ӷ���ʽ2Fe2++H2O2+2H+�T2Fe3++2H2O��
��4����������У�lmol NiSʧȥ6NA�����ӣ�ͬʱ����������ɫ�ж����壮д���÷�Ӧ�Ļ�ѧ����ʽNiS+H2SO4+2HNO3�TNiSO4+SO2��+2NO��+2H2O��
��5�����������У���c��Ni2+��=2.0mol•L-1����ʹ100mL����Һ�е�Ni2+������ȫ[c��Ni2+����10-5mol•L-1]������Ҫ����Na2CO3�������������Ϊ31.4gg��������С�����1λ��Ч���֣�
��6�����������������䣬�ڲ�ͬ�¶��¶Ժ������Ͻ������������������ʱ��仯����ͼ�����������¶���ʱ��ֱ�Ϊ70�桢120min��

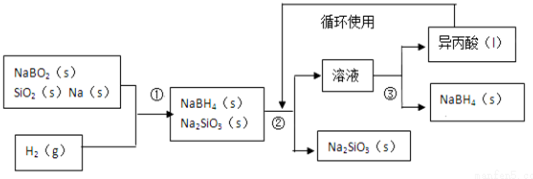
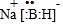 ������BԪ�صĻ��ϼ�Ϊ+3
������BԪ�صĻ��ϼ�Ϊ+3 ��֪A��B��C��D��E��Ϊ��ѧ��ѧ�г������ʣ���һ���������ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ���
��֪A��B��C��D��E��Ϊ��ѧ��ѧ�г������ʣ���һ���������ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ��� ��
��