��Ŀ����
 ij�о���ѧϰС���ͬѧ��ʵ���Ҷ�Cl2ʹ��ɫ������ɫ�Ļ���������̽����
ij�о���ѧϰС���ͬѧ��ʵ���Ҷ�Cl2ʹ��ɫ������ɫ�Ļ���������̽������ͬѧ�������ͼ��ʾ��ʵ��װ�ò�����ʵ�飺
��1����ͬѧʵ���û�еõ�Ԥ�ڵ�ʵ�������������������ʵ��ʧ�ܵ�ԭ��
��2����ͬѧ��ʵ��ʧ�ܺ����Ƶ�ʵ��װ�ý����˸Ľ��������½�����ʵ�飬����õ���Ԥ�ڵ�ʵ����������Ϊ��Ԥ�ڵ�ʵ��������
��3����ͬѧ��Ϊ����Ƶ�ʵ��װ�ü�ʹ�Ľ���ȱ��һװ�ã�ȱ�ٵ�װ���з��������ӷ�Ӧ����ʽΪ
���㣺����ʵ�鷽�������
ר�⣺ʵ�������
��������1��������һ���������壬�ܱ���ʯ�����գ�����ʵ��ʧ�ܣ�
��2����ʵ����иĽ���������ŨH2SO4�����ʯ�ң��������������ʹ�и����ɫ������ɫ��������ʪ�����ɫ�����������ɾ���Ư���ԵĴ����ᣬʹ��ɫ������ɫ��
��3�������ж�������ȱ��β������װ�ã���������������������һ�ص㣬��NaOH��Һ����β������ʣ�
��2����ʵ����иĽ���������ŨH2SO4�����ʯ�ң��������������ʹ�и����ɫ������ɫ��������ʪ�����ɫ�����������ɾ���Ư���ԵĴ����ᣬʹ��ɫ������ɫ��
��3�������ж�������ȱ��β������װ�ã���������������������һ�ص㣬��NaOH��Һ����β������ʣ�
���
�⣺��1����Ϊ������һ���������壬�ܱ���ʯ�����գ����Եò���Ԥ���е�ʪ�����ɫ������ɫ������ʵ��ʧ�ܵ�ԭ����ʹ�õĸ������ʯ�ҽ�Cl2�����ˣ�
�ʴ�Ϊ��ʹ�õĸ������ʯ�ҽ�Cl2���գ�
��2������Ư���Ե��Ǵ����ᣬ���������������������ʺϵĸ����������ͨ����ɫ�����ϵ��Ǹ���������������ڸ������ɫ�����ϲ������ɴ����ᣬ���Ը������ɫ��������ɫ������ʪ�����ɫ�����Ͽ������ɾ���Ư���ԵĴ����ᣬ����ʪ�����ɫ������ɫ���ɴ˵ó�Cl2ʹ��ɫ������ɫ�Ļ�����Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����òŵ�����ɫ������ɫ��
�ʴ�Ϊ���������ɫ��������ɫ��ʪ�����ɫ������ɫ�� Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����ã�
��3�������ж�����������������Ⱦ�����Ա���ȱ��β������װ�ã��������������壬������NaOH��Һ����β����ͼΪ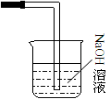 ����Ӧ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
����Ӧ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��ʹ�õĸ������ʯ�ҽ�Cl2���գ�
��2������Ư���Ե��Ǵ����ᣬ���������������������ʺϵĸ����������ͨ����ɫ�����ϵ��Ǹ���������������ڸ������ɫ�����ϲ������ɴ����ᣬ���Ը������ɫ��������ɫ������ʪ�����ɫ�����Ͽ������ɾ���Ư���ԵĴ����ᣬ����ʪ�����ɫ������ɫ���ɴ˵ó�Cl2ʹ��ɫ������ɫ�Ļ�����Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����òŵ�����ɫ������ɫ��
�ʴ�Ϊ���������ɫ��������ɫ��ʪ�����ɫ������ɫ�� Cl2��ˮ��Ӧ���ɴ����ᣬ���������Ư�����ã�
��3�������ж�����������������Ⱦ�����Ա���ȱ��β������װ�ã��������������壬������NaOH��Һ����β����ͼΪ
 ����Ӧ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
����Ӧ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
�����������Ͼ����ʵ������ۺϿ�����������һ�����ԡ��ж������壬����ʹ��ɫ������ɫ����HClO��������������

��ϰ��ϵ�д�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
 �£�H2N-NH2����ƫ������[H2N-N��CH3��2]�����������ȼ�ϣ��������ϵ�֪������һ�����õļ����ܼ������·������ģ�͵ĵ�����������۲죬������ƽ��ͼ��ͼ��ʾ������˵������ȷ���ǣ�������
�£�H2N-NH2����ƫ������[H2N-N��CH3��2]�����������ȼ�ϣ��������ϵ�֪������һ�����õļ����ܼ������·������ģ�͵ĵ�����������۲죬������ƽ��ͼ��ͼ��ʾ������˵������ȷ���ǣ�������| A���·����еĵ�ԭ�Ӳ���sp3�ӻ� |
| B���·����м��м��Լ����зǼ��Լ� |
| C���·����ǷǼ��Է��� |
| D������ƫ�����»���ͬϵ�� |
 �����ķֽⷴӦΪ��COCl2��g���TCl2��g��+CO��g����H��0����Ӧ��ϵ��ƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ��������˵����ȷ���� ��������
�����ķֽⷴӦΪ��COCl2��g���TCl2��g��+CO��g����H��0����Ӧ��ϵ��ƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ��������˵����ȷ���� ��������| A���ӵ�10 min����12 minʱ������Cl2������v��Cl2��=0.01 mol?L-1?min-1 |
| B����8 minʱ���18 minʱ��Ӧ��ƽ�ⳣ����ͬ |
| C������10 minʱ�����ĸı䣬ʹCOCl2��ת���ʽ��� |
| D���ڵ�14 minʱ���ı������������ѹ����Ӧ��������� |
����������ȷ���ǣ�������
| A��pH=2�Ĵ����ˮϡ��100������ҺpHֵ����4 |
| B�������£����ʵ���Ũ����ͬ�ı���������Һ��̼������Һ�ļ���ǿ |
| C����ͬ�¶��£�pH=2���Ȼ�����Һ��ˮ�ĵ���ȱ�pH=1��������Һ��ˮ�ĵ���ȴ� |
| D��pH=3����Һ��pH=11����Һ�������Ϻ���Һһ�������� |
 ������ͼ��ʾװ���Ʊ�������ˮ��̽�����Ʊ�����ˮ����ɺ����ʶ������˿�ѧʵ�飺�ȶ��Ƶõ���ˮ���й۲죬���ý�ͷ�ιܽ�����ˮ��ε���ʢ�к���̪��NaOH��Һ���Թ��У��ߵα����������۲���������Һ�ĺ�ɫ����ȥ������ɫ��Һ���ݴ˻ش�
������ͼ��ʾװ���Ʊ�������ˮ��̽�����Ʊ�����ˮ����ɺ����ʶ������˿�ѧʵ�飺�ȶ��Ƶõ���ˮ���й۲죬���ý�ͷ�ιܽ�����ˮ��ε���ʢ�к���̪��NaOH��Һ���Թ��У��ߵα����������۲���������Һ�ĺ�ɫ����ȥ������ɫ��Һ���ݴ˻ش�