��Ŀ����
17��������ˮ��Һ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ�������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ���һ�֣��������ѧ��ѧ֪ʶ�ش��������⣺��1��AΪ0.1mol•L-1�ģ�NH4��2SO4��Һ���ڸ���Һ������Ũ���ɴ�С��˳��Ϊc��NH4+����c��SO42-����c��H+����c��OH-����
��2��BΪ0.1mol•L-1��NaHCO3��Һ��NaHCO3�ڸ���Һ�д��ڵ�ƽ���У������ӷ���ʽ��ʾ��HCO3-?H++CO32-��HCO3-+H2O?H2CO3+OH-��
��3��CΪ0.1mol•L-1�ģ�NH4��2Fe��SO4��2��Һ����ͬŨ�ȵģ�NH4��2SO4��Һ��Ƚϣ�NH4��2Fe��SO4��2����
���ʵĻ�ѧʽ����Һ��NH4+��Ũ�ȸ�����ԭ����NH4+��Fe2+��ˮ������ԣ�ˮ������ƣ�
��4��Һ���ĵ���������ˮ����д��Һ���ĵ��뷽��ʽ2NH3?NH4++NH2-����Һ���м���NH4Cl��ƽ�⽫���淴Ӧ�����ƶ���
���� ��1�������Ϊǿ�������Σ�笠�����ˮ�����Һ�����ԣ��ٽ�ϵ���غ��ж�����Ũ�ȴ�С��
��2��̼��������Һ�д���̼��������ӵ�ˮ�⡢����ƽ�⣻
��3���������Ӻ�笠�ˮ��������ԣ���������ˮ������笠���ˮ�⣬��NH4��2Fe��SO4��2��Һ��c��NH4+���Դ��Դ˽����⣻
��4������ˮ�ĵ���������Ϣ������������ƽ���ƶ���Ӱ�������
��� �⣺��1�������Ϊǿ�������Σ�笠�����ˮ�����Һ�����ԣ���c��H+����c��OH-�������ݵ���غ��c ��NH4+����c��SO42-����笠�����ˮ��̶Ƚ�С������Һ������Ũ�ȴ�С˳����c��NH4+����c��SO42-����c��H+����c��OH-����
�ʴ�Ϊ��c��NH4+����c��SO42-����c��H+����c��OH-����
��2��̼��������Һ�д���̼��������ӵ�ˮ�⡢����ƽ�⣬�����ӷ���ʽ�ֱ�Ϊ��HCO3-?H++CO32-��HCO3-+H2O?H2CO3+OH-��
�ʴ�Ϊ��HCO3-?H++CO32-��HCO3-+H2O?H2CO3+OH-��
��3����NH4��2Fe��SO4��2��NH4+��Fe2+��ˮ������ԣ�ˮ������ƣ���ˣ�NH4��2Fe��SO4��2��NH4+��ˮ��̶ȣ�NH4��2SO4��ҪС����NH4��2Fe��SO4��2�ȣ�NH4��2SO4��c��NH4+����
�ʴ�Ϊ����NH4��2Fe��SO4��2��NH4+��Fe2+��ˮ������ԣ�ˮ������ƣ�
��4��Һ��������ˮ�ĵ��룬��֪ˮ�ĵ��뷽��ʽ��дΪ2H2O?H3O++OH-����Һ���ĵ��뷽��ʽΪ2NH3?NH4++NH2-����Һ���м���NH4Cl��NH4+Ũ��������ƽ�����淴Ӧ�����ƶ���
�ʴ�Ϊ��2NH3?NH4++NH2-���淴Ӧ����
���� ���⿼��������Ũ�ȴ�С�Ƚϡ�����ˮ��ƽ�⼰��Ӱ�����ص�֪ʶ�㣬��Ҫ������Ũ�ȴ�С�жϡ�����ˮ��Ӱ�����ط�������Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�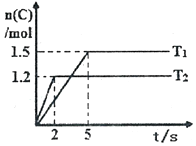 �ֽ�2molA��1molB����2L�ܱ������з�����Ӧ��2A��g��+B��g��?2C��g�����ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
�ֽ�2molA��1molB����2L�ܱ������з�����Ӧ��2A��g��+B��g��?2C��g�����ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | T1��T2 | |
| B�� | �÷�Ӧ���¶�ΪT1ʱ�ﵽƽ��ʱ��������A��C�����ʵ���Ũ����� | |
| C�� | �¶�ΪT2ʱ��2s��B��ƽ������Ϊ0.3mol•L-1•s-l | |
| D�� | �¶�ΪT1ʱ����ƽ��ʱ��Ӧ��A��ת����Ϊ60% |
| ���� | ���� | ���ͻ���� | |
| A | ��ֽ��� Na2SiO3������Һ��Сľ�������ɺ���ھƾ���������� | Сľ����ȼ�� | Na2SiO3������ľ�ķ���� |
| B | �ýྻ��˿պȡ��Һ������ɫ��Ӧ | ����ʻ�ɫ | ��Һ����Na+����K+ |
| C | �ֱ����з�̪���ռ���Һ��ͨ���������������� | ��Һ��ɫ����\ | ˵�������Ͷ��������� Ư���� |
| D | ��ij�ӵ�ʳ����Һ�еμӵ�����Һ | ��Һ��ɫ���� | ��ʳ����һ��û������ KIO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ͬ�����£�H2O������H2��O2�����ʵ���֮��Ϊ2��1 | |
| B�� | ��ͬ�����£�H2O������H2��O2������֮��Ϊ2��1 | |
| C�� | ��ͬ�����£�1molH2��1molO2�������ͬ | |
| D�� | ��ͬ�����£�1molH2��1molO2����������ͬ |
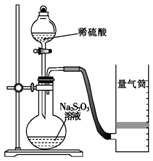 ������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮�ⶨ��ѧ��Ӧ����
��ͬѧ������ͼװ�òⶨ��ѧ��Ӧ���ʣ�
����֪��S2O32-+2H+=H2O+S��+SO2����
��1������ͼ��ʾ��ʵ����Ʒ�������⣬����Ҫ��һ��ʵ�������������
��2������2minʱ�ռ���224mL��������ɱ�״�������壬�ɼ������2min��H+�ķ�Ӧ���ʣ����òⶨֵ��ʵ��ֵƫС����ԭ����SO2�Ჿ������ˮ�����������SO2���ƫС��
��3���Լ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ���ʵ������������ⶨһ��ʱ�����������ʵ�������ⶨһ��ʱ������ҺH+Ũ�ȵı仯�ȣ�дһ�֣���
��Ϊ̽�ֻ�ѧ��Ӧ���ʵ�Ӱ�����أ���Ƶ�ʵ�鷽�����������֪ I2+2S2O32-=S4O62-+2I-������Na2S2O3��Һ��������
| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
| A�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| B�� | �������������ռ���Һ��Ӧ��Cl2+2OH-�TCl-+ClO-+H2O | |
| C�� | ͭ��ϡ���ᷴӦ��Cu+4H++2NO3-�TCu2++2NO2��+2H2O | |
| D�� | ������SO2ͨ��NaOH�У�SO2+2OH-�TSO32-+H2O |
| A�� | ͨ����ѧ�仯����ʵ��16O��18O֮���ת�� | |
| B�� | ��ΪH2O�ķе����H2S������Oԭ�ӵõ�����������Sԭ�� | |
| C�� | ij����������״̬���ܵ��磬�û�������һ�������Ӽ� | |
| D�� | ��ѧ�������ڷ���֮���ǿ�ҵ������ |