��Ŀ����
11������ʵ�������ȷ���ǣ�������| A�� | �������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� | |
| B�� | ����ƿ��©�IJ����ǣ�������ƿ��ע��������ˮ�����ϲ���ƿ����������ָ��סƿ�ף�����ʳָ��סƿ�������ã��۲��Ƿ�©ˮ | |
| C�� | �ڷ�Һ©���з�������Һ��ʱ��Ҫ�ȴ��¶˷ų��ܶȽϴ��Һ�壬�رջ�����ȡ��һֻ�ձ�������ٷų��ܶȽ�С��Һ�� | |
| D�� | ��������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
���� A������ʱ���¶ȼƲⶨ��ֵ��¶ȣ�
B����©ʱ��װˮ�����ã���������ת180�㣬�ظ�����һ�Σ�
C����Һʱ���������²�Һ���ϣ�
D������ʱ�������ɣ��������ȼ��ȣ�
��� �⣺A������ʱ���¶ȼƲⶨ��ֵ��¶ȣ���ʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ�����A��ȷ��
B����©ʱ��������ƿ��ע��������ˮ�����ϲ���ƿ����������ָ��סƿ�ף�����ʳָ��סƿ�������ã��۲��Ƿ�©ˮ����������ת180�㣬�ظ�������������B����
C����Һʱ���������²�Һ���ϣ����ȴ��¶˷ų��ܶȽϴ��Һ�壬�رջ��������Ͽڵ����ϲ�Һ�壬��C����
D������ʱ�������ɣ��������ȼ��ȣ�����ִ�������ʱֹͣ���ȣ���D����
��ѡA��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬���ջ��������ᴿ��ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
1��������һ����Ҫ���л�ԭ�ϣ��ڻ����������������й㷺����;��ijС����װ����װ����ͼ1���Ҵ����������Ũ����Ϊԭ���Ʊ������������ش��������⣮
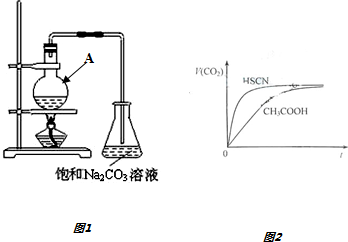
��1������A��������Բ����ƿ��������A�м���ԭ�ϵ���ȷ˳�������ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�ټӱ����ᣮ
��2��������A���������������Ļ�ѧ��Ӧ����ʽΪCH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
��3������������ת���ʣ��ɲ�ȡ�Ĵ�ʩ���Ҵ���������ʱ��������������ȣ�
��4��Ŀǰ�Ը÷�Ӧ�Ĵ����������µ�̽���������������ӣ�H+ ��Һ��������÷�Ӧ�Ĵ����������ظ�ʹ�ã�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ�
���ݱ������ݣ�����C������ĸ��Ϊ�÷�Ӧ�����������
A��120�棬4h B��80�棬2h C��60�棬4h D��40�棬3h
��5�������£���20mL 0.10mol•L-1 CH3COOH��Һ��20mL 10mol•L-1 HSCN��Һ�ֱ���20mL 0.10mol•L-1 NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼ��ͼ2��ʾ����ͼ��������Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ����HSCN�����Ա�CH3COOHǿ������Һ��c��H+���ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ죬��Ӧ��������������Һ�У�c��CH3COO-����c��SCN-�������������������=����
��6�����һ����ʵ��֤�����������ᣮ

��1������A��������Բ����ƿ��������A�м���ԭ�ϵ���ȷ˳�������ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�ټӱ����ᣮ
��2��������A���������������Ļ�ѧ��Ӧ����ʽΪCH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
��3������������ת���ʣ��ɲ�ȡ�Ĵ�ʩ���Ҵ���������ʱ��������������ȣ�
��4��Ŀǰ�Ը÷�Ӧ�Ĵ����������µ�̽���������������ӣ�H+ ��Һ��������÷�Ӧ�Ĵ����������ظ�ʹ�ã�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ�
| ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
| ��Ӧ�¶�/�� | ת���� ��%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���� ��%��* |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
| *ѡ����100%��ʾ��Ӧ���ɵIJ���������������ˮ | |||||
A��120�棬4h B��80�棬2h C��60�棬4h D��40�棬3h
��5�������£���20mL 0.10mol•L-1 CH3COOH��Һ��20mL 10mol•L-1 HSCN��Һ�ֱ���20mL 0.10mol•L-1 NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼ��ͼ2��ʾ����ͼ��������Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ����HSCN�����Ա�CH3COOHǿ������Һ��c��H+���ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ죬��Ӧ��������������Һ�У�c��CH3COO-����c��SCN-�������������������=����
��6�����һ����ʵ��֤�����������ᣮ
20��3.24g������ȫˮ�����������ǵ������ǣ�������
| A�� | 5.04g | B�� | 3.6g | C�� | 6.48g | D�� | 3.24g |