��Ŀ����
�л���M������ʽΪC6H4S4�������ηɻ����������ϵ���Ҫ�ɷ֣�ij��ѧ��ȤС��Ϊ��֤�����Ԫ�أ���̽������ӽṹ����������ʵ�飺
��һ����֤���Ԫ�أ���������Ʒ����A��ȼ�չ��У�ͨ������O2���õ�¯����ʹ����ȼ�գ�����ȼ�ղ�������ͨ������װ�ã����г�������װ������ȥ��
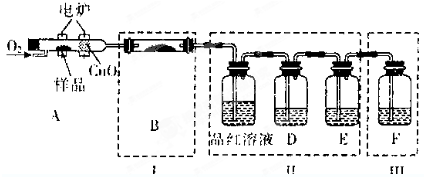
��1��д��A����Ʒȼ�յĻ�ѧ����ʽ ��
��2��װ��B��Ŀ������֤�л����к���Ԫ�أ���B��ʢװ���Լ�Ϊ ��
��3��D��ʢ�ŵ��Լ� ��E��ʢ�ŵ��Լ� ��F��ʢ�ŵ��Լ� ��������ţ��ɹ�ѡ���Լ���a��NaOH��Һ b��Ʒ����Һ c��KMnO4��Һ d��ŨH2SO4 e�����ͳ���ʯ��ˮ
��4����֤���л��ﺬ̼Ԫ�ص������� ��
��5��װ�â��ܻ����������� ��
��6��ȼ�չ��з���CuO�������� ��
��7��ָ��װ��F�Ĵ��� ��
������̽���л���M�ķ��ӽṹ���������ϱ��������л���MΪ������Ԫ���ṹ���кܸߵĶԳ��ԣ���ԭ�ӵĻ�������ͬ����2.04g���л���������CCl4��Һ���������Һ��ɫ����������0.03molBr2��
��8�����л�����ӽṹ�к��еĹ�����Ϊ ����ṹʽ��
��9���л���M�ĽṹʽΪ ������ţ���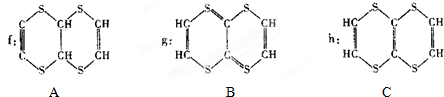
��һ����֤���Ԫ�أ���������Ʒ����A��ȼ�չ��У�ͨ������O2���õ�¯����ʹ����ȼ�գ�����ȼ�ղ�������ͨ������װ�ã����г�������װ������ȥ��

��1��д��A����Ʒȼ�յĻ�ѧ����ʽ
��2��װ��B��Ŀ������֤�л����к���Ԫ�أ���B��ʢװ���Լ�Ϊ
��3��D��ʢ�ŵ��Լ�
��4����֤���л��ﺬ̼Ԫ�ص�������
��5��װ�â��ܻ�����������
��6��ȼ�չ��з���CuO��������
��7��ָ��װ��F�Ĵ���
������̽���л���M�ķ��ӽṹ���������ϱ��������л���MΪ������Ԫ���ṹ���кܸߵĶԳ��ԣ���ԭ�ӵĻ�������ͬ����2.04g���л���������CCl4��Һ���������Һ��ɫ����������0.03molBr2��
��8�����л�����ӽṹ�к��еĹ�����Ϊ
��9���л���M�ĽṹʽΪ

���㣺�ⶨ�л�����ӵ�Ԫ�����
ר�⣺��ѧʵ���뻯ѧ����
��������1��A��Ʒȼ�գ�H��C��SԪ�ص�ȼ�ղ���ֱ���ˮ��������̼�Լ��������ݴ���д����ʽ��
��2��ˮ��ʹ��ɫ����ˮ����ͭ����ɫ��
��3����������Ͷ�����̼����ʹʯ��ˮ����ǣ����������̼֮ǰ�ȼ��������������������������ټ��������̼��
��4����Ԫ�صļ�����Խ���ȼ�ղ�����ˮ�����飬Ҫ�ų�������ˮ�ĸ��ţ�
��5������ͭ���Խ����ɵ�COת����CO2��
��7��û�������ܣ���ʹ��������ѹ������Σ�գ�
��8�����������Ϣ�жϸ��л��ﺬ�еĹ����ţ�
��9�����ݼӳɷ�Ӧ��ԭ���Լ����л���ṹ�ĶԳ����ʽ��н��
��2��ˮ��ʹ��ɫ����ˮ����ͭ����ɫ��
��3����������Ͷ�����̼����ʹʯ��ˮ����ǣ����������̼֮ǰ�ȼ��������������������������ټ��������̼��
��4����Ԫ�صļ�����Խ���ȼ�ղ�����ˮ�����飬Ҫ�ų�������ˮ�ĸ��ţ�
��5������ͭ���Խ����ɵ�COת����CO2��
��7��û�������ܣ���ʹ��������ѹ������Σ�գ�
��8�����������Ϣ�жϸ��л��ﺬ�еĹ����ţ�
��9�����ݼӳɷ�Ӧ��ԭ���Լ����л���ṹ�ĶԳ����ʽ��н��
���
�⣺��1��A��Ʒȼ�գ�H��C��SԪ�ص�ȼ�ղ���ֱ���ˮ��������̼�Լ���������ѧ����ʽΪ��C6H4S4+11O2
6CO2+2H2O+4SO2��
�ʴ�Ϊ��C6H4S4+11O2
6CO2+2H2O+4SO2��
��2����֤�л����к���Ԫ�أ����Ը�����Ԫ��ȼ�ղ�����ˮ������ˮ�Ĵ��ڣ���B��ʢװ���Լ���Ϊ��ˮ����ͭ��
�ʴ�Ϊ����ˮ����ͭ���壻
��3����������Ͷ�����̼����ʹʯ��ˮ����ǣ����������̼֮ǰ�ȼ������������Ʒ����鼴�ɣ�Ȼ����������c�����������ȥ������bƷ����Һ�����Ƿ������������Ȼ����������eʯ��ˮ���������̼��
�ʴ�Ϊ��c��b��e��
��4��̼Ԫ��ȼ�ղ����Ƕ�����̼��������̼�����ó���ʯ��ˮ�����飬���������̼֮ǰ�ȼ������������Ʒ����鼴�ɣ�Ȼ���������ø����������ȥ��������������ʯ��ˮ���������̼��
�ʴ�Ϊ��E��Ʒ����Һ����ɫ��F����Һ����ǣ�
��5����Ԫ�صļ�����Խ���ȼ�ղ�����ˮ�����飬��һ��Ҫ�ų�������ˮ�ĸ��ţ�����װ�â��ܻ�����
�ʴ�Ϊ������ͨ����ʱ�������ˮ������������Ԫ�ص���֤��
��6������ͭ���Խ�̼Ԫ�ز���ȫȼ�����ɵ�COת����CO2��
�ʴ�Ϊ�����л����е�̼Ԫ��ȫ�������ɶ�����̼��
��7��װ��Fֻ�н�����û�������ܣ���ʹ��������ѹ������Σ�գ�
�ʴ�Ϊ��û�������ܣ���ʹ��������ѹ������Σ�գ�
��8��2.04g���л�������ʵ���Ϊ��
=0.01mol��0.01mol���л���������CCl4��Һ���������Һ��ɫ����������0.03molBr2�����Ը��л����к���3mol��̼̼˫����������MΪ������Ԫ���ṹ���кܸߵĶԳ��ԣ���ԭ�ӵĻ�������ͬ�����A��B��C�Ľṹ��ʽ��ֻ��C���ϣ�
�������Ϸ�����֪�����л�������к��й�����̼̼˫����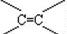 ��
��
�ʴ�Ϊ��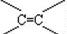 ��
��
��9���л���A��B�Ľṹ��ʽ�в����жԳ��ԣ���ԭ�ӵ�λ�ò���ȫ��ͬ��ֻ��C���������������Ը��л���Ľṹ��ʽΪC��
�ʴ�Ϊ��C��
| ||
�ʴ�Ϊ��C6H4S4+11O2
| ||
��2����֤�л����к���Ԫ�أ����Ը�����Ԫ��ȼ�ղ�����ˮ������ˮ�Ĵ��ڣ���B��ʢװ���Լ���Ϊ��ˮ����ͭ��
�ʴ�Ϊ����ˮ����ͭ���壻
��3����������Ͷ�����̼����ʹʯ��ˮ����ǣ����������̼֮ǰ�ȼ������������Ʒ����鼴�ɣ�Ȼ����������c�����������ȥ������bƷ����Һ�����Ƿ������������Ȼ����������eʯ��ˮ���������̼��
�ʴ�Ϊ��c��b��e��
��4��̼Ԫ��ȼ�ղ����Ƕ�����̼��������̼�����ó���ʯ��ˮ�����飬���������̼֮ǰ�ȼ������������Ʒ����鼴�ɣ�Ȼ���������ø����������ȥ��������������ʯ��ˮ���������̼��
�ʴ�Ϊ��E��Ʒ����Һ����ɫ��F����Һ����ǣ�
��5����Ԫ�صļ�����Խ���ȼ�ղ�����ˮ�����飬��һ��Ҫ�ų�������ˮ�ĸ��ţ�����װ�â��ܻ�����
�ʴ�Ϊ������ͨ����ʱ�������ˮ������������Ԫ�ص���֤��
��6������ͭ���Խ�̼Ԫ�ز���ȫȼ�����ɵ�COת����CO2��
�ʴ�Ϊ�����л����е�̼Ԫ��ȫ�������ɶ�����̼��
��7��װ��Fֻ�н�����û�������ܣ���ʹ��������ѹ������Σ�գ�
�ʴ�Ϊ��û�������ܣ���ʹ��������ѹ������Σ�գ�
��8��2.04g���л�������ʵ���Ϊ��
| 2.04g |
| 204g/mol |
�������Ϸ�����֪�����л�������к��й�����̼̼˫����
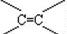 ��
���ʴ�Ϊ��
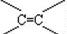 ��
����9���л���A��B�Ľṹ��ʽ�в����жԳ��ԣ���ԭ�ӵ�λ�ò���ȫ��ͬ��ֻ��C���������������Ը��л���Ľṹ��ʽΪC��
�ʴ�Ϊ��C��
���������⿼�����л��������Ԫ�ص���ɡ��л���ṹ�����ʣ���Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ���ȷ�ж��л���������Ԫ�صķ���������������ѧ���ķ���������������

��ϰ��ϵ�д�
�����Ŀ
�����±����е����ܵ����ݣ������ж��д�����ǣ���������
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4600 | 6900 | 9500 |
| Y | 580 | 1800 | 2700 | 11600 |
| A��Ԫ��X��Y������ͬ����Ԫ�� |
| B��Ԫ��X�������Ǣ�A��Ԫ�� |
| C��Ԫ��X�����γɻ�����ʱ����ѧʽ������XCl |
| D��Ԫ��Y�ڻ�ѧ�������������� |
�����������ʵ��ó��Ľ�����ȷ���ǣ�������
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ����̼������Һ |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һ��һ����������Һ |
| C����ijδ֪��Һ�м���ϡNaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�������������ԭ��Һ��û��NH4+ |
| D��ij��Һ�м���BaCl2��Һ������������ϡ����İ�ɫ����������Һһ������Ag+ |
�����ܼ����Ų���ȷ���ǣ�������
| A��6S | B��5p | C��4d | D��3f |
 A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���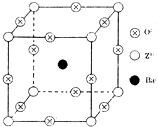 ��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��