��Ŀ����
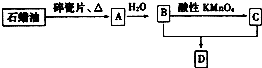 A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ�����l����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����
��2��A�Ļ�ѧʽ��
��3����������ʯ���ͻ��A�Ĺ����е��м����֮һ��д����������ͬ���칹��Ľṹ��ʽ��
���㣺�л�����ƶ�,�л���Ľṹ������
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������A�IJ���ͨ������һ�����ҵ�ʯ�ͻ���ˮƽ����AӦΪCH2=CH2����ˮ��һ�������·����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ������Ը��������������CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����DΪCH3COOCH2CH3���Դ˽����⣮
���
�⣺A�IJ���ͨ������һ�����ҵ�ʯ�ͻ���ˮƽ����AӦΪCH2=CH2����ˮ��һ�������·����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ������Ը��������������CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����DΪCH3COOCH2CH3��
��1����ҵ�ϣ���ʯ�ͷ�����ʯ���ͣ�ʯ���;��ѽ�ɵõ���ϩ��Ϊ��ѧ�仯���ʴ�Ϊ������ѧ��
��2��������������֪��A�Ļ�ѧʽΪC2H4��A��B��CH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����ӦB+C��D���Ҵ���������Ũ���ᡢ��������������������������Ӧ����ʽ�ǣ�CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��C2H4���ӳɣ�CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
��3����������������춡������ͬ���칹�壬�ṹ��ʽ�ֱ�Ϊ��CH3CH2CH2CH3�� ��
��
�ʴ�Ϊ��CH3CH2CH2CH3�� ��
��
��1����ҵ�ϣ���ʯ�ͷ�����ʯ���ͣ�ʯ���;��ѽ�ɵõ���ϩ��Ϊ��ѧ�仯���ʴ�Ϊ������ѧ��
��2��������������֪��A�Ļ�ѧʽΪC2H4��A��B��CH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����ӦB+C��D���Ҵ���������Ũ���ᡢ��������������������������Ӧ����ʽ�ǣ�CH3CH2OH+CH3COOH
| Ũ���� |
| �� |
�ʴ�Ϊ��C2H4���ӳɣ�CH3CH2OH+CH3COOH
| Ũ���� |
| �� |
��3����������������춡������ͬ���칹�壬�ṹ��ʽ�ֱ�Ϊ��CH3CH2CH2CH3��
 ��
���ʴ�Ϊ��CH3CH2CH2CH3��
 ��
��
���������⿼���л�����ƶϣ��漰ϩ�봼�����ᡢ����֮���ת����ϵ�ȣ�AΪ��ϩΪ�ƶϵ�ͻ�ƿڣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
�����Ŀ
ijԭ�ӵ����������Ų�Ϊnsnnp2n+1�����Ԫ�������ڱ��е�λ��Ϊ��������
| A���ڶ����ڣ���A�� |
| B���ڶ����ڣ���A�� |
| C���������ڣ���A�� |
| D���������ڣ���A�� |
��������Ϊ����Ԫ�صĺ˵����������ԭ�Ӻ������������������ǣ�������
| A��8 | B��14 | C��17 | D��19 |
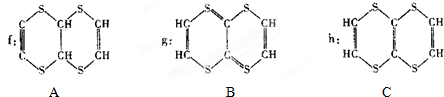

 ��Ϊͬϵ�����
��Ϊͬϵ�����



 ��֪������ͭ��Һ�е��백�������ƣ�H2N-CH2-COONa�����ɵõ������A����ṹ��ͼ1��
��֪������ͭ��Һ�е��백�������ƣ�H2N-CH2-COONa�����ɵõ������A����ṹ��ͼ1�� ijͬѧ����ͼ1��ʾ��װ����̽��SO2�����ʼ��й�ʵ�飮
ijͬѧ����ͼ1��ʾ��װ����̽��SO2�����ʼ��й�ʵ�飮