��Ŀ����
19������������һ���º͵���������������Ϊ��ɫ���壬�������ᡢˮ���Ҵ����ܼ���ijʵ��С����ѡ����ͼ1װ�ã����̶ֹ�װ���ԣ��Ʊ��������ƣ�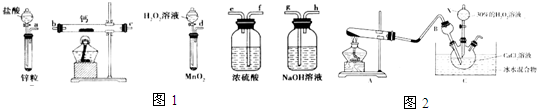
��1����ѡ���Ҫ��װ�ã���������������˳��Ϊdfebcf��dfecbf�����������ӿڵ���ĸ��ţ�װ�ÿ��ظ�ʹ�ã�
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£��ټ��װ�������Ժ�װ��ҩƷ���ڴ�Һ©��������ͨ��һ��ʱ�����壬����ҩƷ���۷�Ӧ������Ϩ��ƾ��ƣ�����Ӧ����ȴ�����£�ֹͣͨ�����������رշ�Һ©���Ļ�����������������ܲ��װ�ã�ȡ�����
��3�����÷�ӦCa2++H2O2+2NH3+8H2O=CaO2•8H2O��+2NH4+���ڼ��Ի�������ȡCaO2•8H2O��װ����ͼ2��
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��NH3��Ca2+��H2O2�ķ�Ӧ������������������к��������Ca2+��Ӧ�����������ӣ��ٽ���Ӧ���У���ʹ��Һ�ʼ��ԣ�����CaO2•8H2O���ܽ⣬����߲�Ʒ�IJ��ʣ���
�۷�Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2•8H2O������CaO2•8H2O�Ƿ�ϴ�Ӹɾ��IJ���Ϊȡ����ϴ��Һ�������Թ��У��ȼ���ϡ�����ữ���ٵμ���������Һ����û�а�ɫ�������ɣ���˵���Ѿ�ϴ�Ӹɾ�����֮��˵��δϴ�Ӹɾ���
����֪CaO2��350��Ѹ�ٷֽ�����CaO��O2������ȡ��Ʒ������Ϊm g������������ʱ��ʣ�����n g�����Ʒ��CaO2����������Ϊ$\frac{9��m-n��}{2m}$��100%������ĸ��ʾ����
���� ��1��һ���Ʊ���������������ʵ��װ�õ�˳��Ϊ���Ʊ�װ�á�����װ�á�����װ�õȣ�
��2��ʵ�����ʱΪ��ֹ��������װ�ã���Ҫ����ͨ����ֱ��װ����ȴ��
��3���ٸ���װ��ͼ��֪��A�����Ȼ�����������Ƽ����ư������ݴ���д��ѧ����ʽ��
�����ڹ��������Ca2+��Ӧ����������ӣ��ð����к������ӣ����Դ�ʹ��Ӧ������У�
�۷�Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2.8H2O��ͨ������ϴ����Һ���Ƿ����������ж�CaO2.8H2O�Ƿ�ϴ�Ӹɾ���
�ܽ�������֪������������Ϊmg-ng�����ݷ�Ӧ2CaO2$\frac{\underline{\;350��\;}}{\;}$2CaO+O2������ù������Ƶ�����������ȷ������������
��� �⣺��1���Ʊ���������ʱ����˫��ˮ�Ʊ������������ڼ����õĽ�����������ˮ��Ӧ�����������ƺ����������Ʊ��������л����ˮ��������������ƻ���֮ǰ��Ҫ���ѡ���Լ���Ũ���ͬʱΪ��ֹ������ˮ�������룬�����Ҫ����Ũ�����ϴ��ƿ��������ȷ��˳��Ϊ��dfebcf��dfecbf��
�ʴ�Ϊ��dfebcf��dfecbf��
��2��ʵ�����ʱΪ��ֹ��������װ�ã���Ҫ����ͨ����ֱ��װ����ȴ������ʵ�����ʱ�IJ���ΪϨ��ƾ��ƣ�����Ӧ����ȴ�����£�ֹͣͨ�����������رշ�Һ©���Ļ�����
�ʴ�Ϊ��Ϩ��ƾ��ƣ�����Ӧ����ȴ�����£�ֹͣͨ�����������رշ�Һ©���Ļ�����
��3���ٸ���װ��ͼ��֪��A�����Ȼ�����������Ƽ����ư�������Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�����ڹ��������Ca2+��Ӧ����������ӣ��ð����к������ӣ����Դ�ʹ��Ӧ������У�������������Ϊ�к��������Ca2+��Ӧ�����������ӣ��ٽ���Ӧ���У���ʹ��Һ�ʼ��ԣ�����CaO2•8H2O���ܽ⣬����߲�Ʒ�IJ��ʣ���
�ʴ�Ϊ���к��������Ca2+��Ӧ�����������ӣ��ٽ���Ӧ���У���ʹ��Һ�ʼ��ԣ�����CaO2•8H2O���ܽ⣬����߲�Ʒ�IJ��ʣ���
�۷�Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2.8H2O��ͨ������ϴ����Һ���Ƿ����������ж�CaO2.8H2O�Ƿ�ϴ�Ӹɾ�������Ϊȡ����ϴ��Һ�������Թ��У��ȼ���ϡ�����ữ���ٵμ���������Һ����û�а�ɫ�������ɣ���˵���Ѿ�ϴ�Ӹɾ�����֮��˵��δϴ�Ӹɾ���
�ʴ�Ϊ��ȡ����ϴ��Һ�������Թ��У��ȼ���ϡ�����ữ���ٵμ���������Һ����û�а�ɫ�������ɣ���˵���Ѿ�ϴ�Ӹɾ�����֮��˵��δϴ�Ӹɾ���
�ܽ�������֪������������Ϊmg-ng�����ݷ�Ӧ2CaO2$\frac{\underline{\;350��\;}}{\;}$2CaO+O2������ù������Ƶ�����Ϊ��m-n��g��$\frac{72��2}{32}$=4.5��m-n��g�����Բ�Ʒ��CaO2����������Ϊ��$\frac{4.5��m-n��g}{mg}$��100%=$\frac{9��m-n��}{2m}$��100%��
�ʴ�Ϊ��$\frac{9��m-n��}{2m}$��100%��
���� ���⿼��ʵ�鷽������ơ�������ɼ������ⶨ���㣬��Ŀ�Ѷ��еȣ���ȷʵ��Ŀ�ĺ�ʵ��ԭ��Ϊ���ؼ���ע����������ʵ�鷽����Ƶ�������ԭ����ȷ̽��������ɼ������ⶨ�ķ����������ֿ���ѧ���ķ�������������֪ʶǨ��Ӧ��������


| A�� | ������ӦΪ��Zn-2e-�TZn2+ | |
| B�� | ��ط�ӦΪ��Zn+Cu2+�TZn2++Cu | |
| C�� | �����·�У����Ӵ�ͭ�缫����п�缫 | |
| D�� | �����е�K+����ZnSO4��Һ |
| A�� | ����ƿ��ʹ��֮ǰҪ��© | |
| B�� | ����ƿ�����¶ȣ�Ũ�ȣ�����������ʶ | |
| C�� | ����ʱ������ʹҺ���������ƿ�Ŀ̶��ߣ����õιܽ�����Һ������ | |
| D�� | ���úõ���Һ����������ƿ�У������ϱ�ǩ |
��1������β����������Ҫԭ��Ϊ��
2NO��g��+2CO$?_{����}^{����}$2CO2��g��+N2��g����H��O
�ٸ÷�Ӧ������ʱ��������ͼ����ͼ��ʾ���������������䣬���ڷ�Ӧǰ������ʵĴ�����������ʱ��ͼ����ͼ����ͼ��ʾ������˵����ȷ����BC�����Ӧ��ĸ����

A��a1��a2��B��b1��b2 C��t1��t2 D����ͼ����Ӱ����������� E����ͼ����Ӱ�����������
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����BD������ţ���

��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⣮úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
��֪��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-867kJ/mol
2NO2��g���TN2O4��g����H=-56.9kJ/mol H2O��g��=H2O��l����H=-44.0kJ/mol
д��CH4����ԭN2O4��g������N2��H2O��l�����Ȼ�ѧ����ʽ��CH4��g��+N2O4��g���TN2��g��+2H2O��l��+CO2��g����H=-898.1kJ/mol��
��3��CH4��H2O��g�� �ڴ������淢����ӦCH4+H2O?CO+3H2���÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ�����±���
| �¶�/�� | 800 | 1000 | 1200 | 1400 |
| ƽ�ⳣ�� | 0.45 | 1.92 | 276.5 | 1771.5 |
��T��ʱ����1L�ܱ�������Ͷ��l mol CH4��l mol H2O��g����ƽ��ʱc��CH4��=0.5mol/L�����¶��·�ӦCH4+H2O�TCO+3H2��ƽ�ⳣ��K=6.75��
��4������ȼ�ϵ�ؿ����������������ʣ���ͼ�����ü���ȼ�ϵ�ص��100ml1mol/Lʳ��ˮ�����һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩��

�ټ���ȼ�ϵ�صĸ�����Ӧʽ��CH4-8e-+2H2O=CO2+8H+
�ڵ�����Һ��pH=14����������������������Һ��Ӧ��
�������������������ڱ�״������1.68L��

| A�� | ����Mg����������������Mg��ϡ�����Al | |
| B�� | ����Al��������OH?Ǩ�Ʒ���Al�����·��Mg | |
| C�� | ����Fe���������缫��Ӧʽ��2H++2e?�TH2�� | |
| D�� | ����Cu���������缫��Ӧʽ��O2+4e?+2H2O�T4OH? |
 ij̽��С�齫��ӦCu��s��+2Ag+��aq���TCu2+��aq��+2Ag ��s����Ƴ�ԭ��أ�ijʱ�̵ĵ�������������A��ָ��ƫת������ͼ��ʾ��
ij̽��С�齫��ӦCu��s��+2Ag+��aq���TCu2+��aq��+2Ag ��s����Ƴ�ԭ��أ�ijʱ�̵ĵ�������������A��ָ��ƫת������ͼ��ʾ�� �����£���FeCl3��Һ��KI��Һ��ϣ��������·�Ӧ��2Fe3++2I-?2Fe2++I2
�����£���FeCl3��Һ��KI��Һ��ϣ��������·�Ӧ��2Fe3++2I-?2Fe2++I2 ��1��ij����С��ͬѧ��ͼ1װ�ý���ʵ�飬�Իش��������⣺
��1��ij����С��ͬѧ��ͼ1װ�ý���ʵ�飬�Իش��������⣺ A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ�
A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ� ��
��