��Ŀ����
12���ش��������⣮��1��Ũ������ľ̿�ڼ��������·�Ӧ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2+SO2+2H2O
��2��������ͼ���и���װ�����һ��ʵ������֤������Ӧ�������ĸ��ֲ���
| ��� | �� | �� | �� | �� |
| װ�� |  |  |  |  |
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫ��Cƿ����Һ����ɫ��Aƿ��Һ����������֤�������Ƿ��ж�������Bƿ��Һ�������dz�ȥ��������Cƿ��Һ��������ȷ�������ж��������Ѿ���Bƿ��Һ������
��4��װ�â������ӹ���ҩƷ����ˮ����ͭ������֤�IJ�����H2O��ȷ��װ�â�������װ����λ�õ����������ڲ�������ͨ��װ��1��3����ˮ����������2������1��3֮ǰ��
��5��װ�â�����ʢ��Һ�dz���ʯ��ˮ������֤�IJ����Ƕ�����̼��
���� ��1�����������£�ľ̿��Ũ���ᷢ��������ԭ��Ӧ���ɶ�����̼�����������ˮ��
��2��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ������������ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ����ʹƷ����Һ��ɫ������Ư���ԣ���������̼û�д����ʣ����Լ��������̼֮ǰ�ȼ����������ˮ��������ˮ����ͭ���飬��������˳���ǣ������Ʊ���ˮ�����������������������ȥ���������������������������̼���飻
��3��A�������Ǽ����������B�������dz�ȥ��������C�������Ǽ�����������Ƿ���ڣ�
��4��ˮ������ʹ��ˮ����ͭ����ɫ��Ϊ��ɫ����������ˮ����ͭ����ˮ������װ����Ҫ�ڿ�ʼ����ˮ�Ĵ��ڣ��������Һ��ͨ�������ˮ������
��5��ʵ�����ó���ʯ��ˮ���������̼��
��� �⣺��1�����������£�ľ̿��Ũ���ᷢ��������ԭ��Ӧ���ɶ�����̼�����������ˮ����Ӧ����ʽΪC+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
��2��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ������������ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ����ʹƷ����Һ��ɫ������Ư���ԣ���������̼û�д����ʣ����Լ��������̼֮ǰ�ȼ����������ˮ��������ˮ����ͭ���飬��������˳���ǣ������Ʊ���ˮ�����������������������ȥ���������������������������̼���飬����������˳���Ǣܡ��ڡ��١��ۣ�
�ʴ�Ϊ���ܣ��ڣ��٣��ۣ�
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫcƿ����Һ����ɫ��˵��ͨ��cƿ�������в�����������
A�������Ǽ��������������Ʒ����Һ��ɫ�����������
����������л�ԭ�ԡ����Ը�����ؾ���ǿ�����ԣ����߷���������ԭ��Ӧ��ʹ���Ը��������Һ��ɫ������B�������dz�ȥ��������
C�������Ǽ�����������Ƿ���ڣ���ֹ���Ŷ�����̼���飬
�ʴ�Ϊ����֤�������Ƿ��ж�������ȥ��������ȷ�������ж��������Ѿ���Bƿ��Һ������
��4��ˮ������ʹ��ˮ����ͭ����ɫ��Ϊ��ɫ����������ˮ����ͭ����ˮ����������װ�â�����װ�Ĺ���ҩƷ����ˮ����ͭ��Ҳ��֤�IJ�����ˮ�����ڲ�������ͨ��װװ1��3����ˮ����������2������1��3֮ǰ��
�ʴ�Ϊ����ˮ����ͭ��H2O�����ڲ�������ͨ��װ��1��3����ˮ����������2������1��3֮ǰ��
��5��������̼��ʹ����ʯ��ˮ����ǣ�����ʵ�����ó���ʯ��ˮ���������̼����װ�â�����ʢ��Һ�dz���ʯ��ˮ��
�ʴ�Ϊ������ʯ��ˮ��������̼��
���� ������Ũ����Ϊ���忼������ʵ�鷽����ƣ���ȷʵ��ԭ���ǽⱾ��ؼ���֪�����ʼ����Ⱥ�˳�����Լ���ѡȡ�������ѵ���ʵ��װ������˳����Ŀ�Ѷ��еȣ�

| A�� | ���� | B�� | ʳ�� | C�� | ��� | D�� | ���� |
| A�� | ������SO2 ͨ��NaOH��Һ�У�SO2+2OH-�TSO32-+H2O | |
| B�� | NaNO2��Һ�м�������KMnO4��Һ��2MnO4-+5NO2-+6H+�T2Mn2++5NO3-+3H2O | |
| C�� | ����������Һ�����������Һ��ϣ�Fe2++2H2O2+4H+�TFe3++4H2O | |
| D�� | NaHCO3��Һ�м������Ba��OH��2��Һ��2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32 |
| A�� |  ȩ�� ȩ��  | B�� |  ����-OH ����-OH | C�� |  ȩ�� ȩ��  | D�� | CH3COOH ������  |
| A�� | �������1�����ӵ�ԭ�� | B�� | ���������Ų�Ϊns2��ԭ�� | ||
| C�� | �������3��δ�ɶԵ��ӵ�ԭ�� | D�� | �������δ�ɶԵ��ӵ�ԭ�� |

| A�� | ͼ1װ�������к��ȵIJⶨ | |
| B�� | ͼ2װ�����ڸ��������Һ�ζ����� | |
| C�� | ͼ3װ�����ڲⶨ�����ķ�Ӧ���ʣ���λmL/s�� | |
| D�� | ͼ4װ�������о���ͬ�����Է�Ӧ���ʵ�Ӱ�� |
| A�� | CO2 | B�� | C2H2 | C�� | H2O2 | D�� | COCl2 |
 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺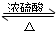 CH3COOC2H5+H2O
CH3COOC2H5+H2O