��Ŀ����
2��ij��ѧ��ȤС��ⶨij����������Һ�й��������Ũ�ȣ���������ʵ�飺ȡ20.00mL�ĸù���������Һ��ˮϡ����250.00mL��ȡϡ�ͺ�Ĺ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͣ��������������ø�����ر���Һ�ζ������������䷴Ӧ�����ӷ���ʽΪ��2MnO4-+5H2O2+6H+�T2Mn2++8H2O+5O2�����ݴ�����
��1���ζ�ʱ����������ر���Һע����ʽ�����ʽ����ʽ�����ζ����У��ζ������յ�������ǣ��������һ�θ�����ر�Һ����ƿ����Һ����ɫ���Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2�����ñ���Һʱʹ�õ�KMnO4�����������������K2SO4���ʣ���Բⶨ�����Ӱ����ƫ���ƫ����ƫС�����䡱��
��3���ظ��ζ��ĴΣ��ֱ����c mol/LKMnO4����Һ�����Ϊ��0.99V mL��1.03V mL��1.28V mL��0.98V mL����ԭ����������Һ�е����ʵ���Ũ��Ϊ1.25cVmol•L-1��
���� ��1��������ؾ���ǿ�����ԣ���������Ƥ��������ر�����һ���Ϻ�ɫ��Һ�壬���ﵽ�ζ��յ�ʱ������ͨ��������ɫ���жϣ�
��2�����ñ���Һʱʹ�õ�KMnO4�����������������K2SO4���ʣ���ҺŨ��ƫС��
��3���ȸ����������Ч��ȷ�������ƽ��ֵ���ٸ��ݹ�ϵʽ��2MnO4-��5H2O2������������Ũ�ȣ�
��� �⣺��1�����ڸ�����ر���Һ����ǿ�����ԣ���������Ƥ�������ü�ʽ�ζ��ܣ�����ֻ��ʹ����ʽ�ζ��ܣ�
�ζ������յ�������ǣ��������һ�θ�����ر�Һ����ƿ����Һ����ɫ���Ϻ�ɫ���Ұ���Ӳ���ɫ��
�ʴ�Ϊ����ʽ���������һ�θ�����ر�Һ����ƿ����Һ����ɫ���Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2�����ñ���Һʱʹ�õ�KMnO4�����������������K2SO4���ʣ���ҺŨ��ƫС��������ĵ����ƫ��ʹ�ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ��
��3���Ĵ����ı���Һ�����Ϊ��0.99V mL��1.03V mL��1.28V mL����ȥ����0.98V mL���������ε�ƽ��ֵΪ1.00V mL
2MnO4-��5H2O2
2 5
c mol/L��1.00V mL��$\frac{250}{25}$ C��H2O2����20.00mL
��ã�C��H2O2��=1.25cV��
�ʴ�Ϊ��1.25cV��
���� ���⿼����������ԭ�ζ������㣬����ʵ���ԭ���Ǽ���Ĺؼ���Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

| A�� | CaSiO3���� | B�� | SiO2 | C�� | Na2SiO3ˮ��Һ | D�� | H2SiO3 |
| A�� | ���ܺ���Ag+��Al3+��NH${\;}_{4}^{+}$ | |
| B�� | һ������Cl-�����ܺ���NO${\;}_{3}^{-}$ | |
| C�� | һ������AlO${\;}_{2}^{-}$��NH${\;}_{4}^{+}$��CO${\;}_{3}^{2-}$ | |
| D�� | ���ܺ���Fe3+��Fe2+��һ������AlO${\;}_{2}^{-}$��CO${\;}_{3}^{2-}$ |
| A�� | ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ��� | |
| B�� | NaOH�����ܽ������ת������ƿ | |
| C�� | ������ƿ�н��ж���ʱ���ӿ̶��� | |
| D�� | ���ݺ������ƿ��תҡ�ȣ�����Һ����ڿ̶ȣ��ٲ��伸��ˮ���̶� |
��1��Ũ������ľ̿�ڼ��������·�Ӧ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2+SO2+2H2O
��2��������ͼ���и���װ�����һ��ʵ������֤������Ӧ�������ĸ��ֲ���
| ��� | �� | �� | �� | �� |
| װ�� |  |  |  |  |
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫ��Cƿ����Һ����ɫ��Aƿ��Һ����������֤�������Ƿ��ж�������Bƿ��Һ�������dz�ȥ��������Cƿ��Һ��������ȷ�������ж��������Ѿ���Bƿ��Һ������
��4��װ�â������ӹ���ҩƷ����ˮ����ͭ������֤�IJ�����H2O��ȷ��װ�â�������װ����λ�õ����������ڲ�������ͨ��װ��1��3����ˮ����������2������1��3֮ǰ��
��5��װ�â�����ʢ��Һ�dz���ʯ��ˮ������֤�IJ����Ƕ�����̼��


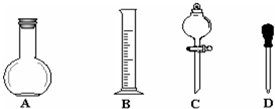 ʵ��������1000mL 0.1mol•L-1��Na2CO3��Һ����ش��������⣺
ʵ��������1000mL 0.1mol•L-1��Na2CO3��Һ����ش��������⣺