��Ŀ����
1��ijС��ͬѧ̽��H2O2��H2SO3��Br2������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ��飩����ش��������⣺
��1��д������A�����ƣ����������ܣ�

��2��ʵ���¼���£�
| ���� | ʵ����� | ʵ������ | ʵ����� |
| �� | ����a����μ���H2SO3��Һ������ | ��ƿ����Һ�ɳȻ�ɫ��Ϊ��ɫ | Br2�������Դ���H2SO3 |
| �� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | H2O2�������Դ���Br2 |
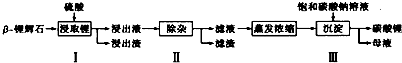
��4��ʵ���ҳ���Cl2ͨ��NaBr��Һ���Ƶõ����壬��ҵ�ϳ��õ�ⱥ��ʳ��ˮ�Ʊ�Cl2��װ����ͼ��ʾ�����ø�װ�ã���ҵ�ϳ��õ������⣬�����Եõ�NaOH��H2���ѧʽ����װ���е����ӽ���ĤNa+����ͨ����OH-����ͨ������Ŀ���DZ���Cl2��NaOH��Ӧ����NaClO��Ӱ��NaOH�IJ����ʹ��ȣ�

���� �����������Է���������ԭ��Ӧ���������������Դ������������ԭ�����ʵ�飬̽��H2O2��H2SO3��Br2������ǿ�����漰ʶ������A�����ƣ���Ϸ�Ӧԭ������ʵ������п��ܳ��ֵ����������ӷ���ʽ��ʾ��Ӧԭ��������������ȼҵ��������ԭ����
��1����Ӧ�����Ϸ�����A�������������ݳ����������ɳ�������ʶ���A�����ƣ�
��2�����ݽ���Br2�������Դ���H2SO3��֪����ˮ��ǿ�����ԣ�������H2SO3������H2SO3��Һ��μ��룬��Һ���������С�������Һ����ɫ���dzֱ����ɫ����������õķ�Ӧ��Һ����μ���H2O2��Һ����Һ�ֱ�Ϊ�Ȼ�ɫ���ɼ���Һ���Br-�ֱ�����ΪBr2��˵����H2O2�����Դ���Br2��
��3����Ϊ�����μӵ�H2SO3��Һ�ǹ����ģ���ʼ����H2O2��Һʱû��ֱ��������Һ���Br-���������������H2SO3������������ԭ��Ӧ���ʲ�����е����H2O2�ֱ���H2SO3��Br-�����˷�Ӧ��
��4�����װ��Ϊ��ⱥ��ʳ��ˮ�ĵ��أ��������ȼҵ��������ɵõ��������������ƣ����ӽ���Ĥ��������OH-����Ϊ�����OH-�����ɵ��������������ܽ��ڼ�����Һ��Ƶõ��������Ʋ�����
��� �⣺��1������AΪ�����Ļ�ѧ������������ʶ��ӦΪ���������ܣ��ʴ�Ϊ�����������ܣ�
��2����Ӧ�����еμ�H2SO3��Һ����ˮ�е���ᱻ��ԭΪBr-����ˮΪ�Ȼ�ɫ�����ŵμӵ�H2SO3��Һԭ��Խ�࣬��Һ�����Խ��Խ�٣���ɫ����dz��������ɫ���������������H2O2��Һ���ʸտ�ʼ��Һ��ɫû�б仯�������μ���Һ��Ϊ�Ȼ�ɫ��˵��H2O2��������Һ���Br-����Br2��������Ӧ�Ļ�ѧ����ʽΪ2H2O2+2HBr=Br2+2H2O������������ԭ��Ӧ��֪H2O2�������Դ���Br2��
�ʴ�Ϊ���ɳȻ�ɫ��Ϊ��ɫ��H2O2�������Դ���Br2��
��3���������������H2O2��Һ������H2SO3����H2SO3��ȫ��������������Һ���Br-��������Һ���ɫ�����ձ�Ȼ�ɫ�������ķ�Ӧ���ӷ���ʽΪH2SO3+H2O2=2H++SO42-+H2O��2H++2Br-+H2O2=Br2+2H2O��
�ʴ�Ϊ������1��H2SO3�й�����H2O2�Ⱥ�H2SO3��Ӧ��H2SO3+H2O2=2H++SO42-+H2O��2H++2Br-+H2O2=Br2+2H2O��
��4���ȼҵ�õ��IJ������������������������ƣ����OH-������Ĥ���������������ɵ��������������ܽ��ڼ�����Һ��õ����������Ʋ�������Ӱ�������
�ʴ�Ϊ��NaOH��H2������Cl2��NaOH��Ӧ����NaClO��Ӱ��NaOH�IJ����ʹ��ȣ�
���� ������������ԭ��Ӧ���������������Դ�����������Ϊ���壬̽��H2O2��H2SO3��Br2������ǿ����������ʵ��Ļ���������������ѧ�����ʵ���������ԭ������������һ�����Ѷȣ�

| A�� | ����������һ���ǽ��������� | |
| B�� | ����������һ���Ǽ��������� | |
| C�� | �������������������һ��������ˮ��Ӧ������Ӧ���ᡢ�� | |
| D�� | ����������һ���Ƿǽ��������� |
| A�� | ��ͨп�̸ɵ��̼���Ǹ�����пƬ������ | |
| B�� | ʢˮ���������ڿ�����ˮ���紦��������ʴ | |
| C�� | Ϊ��ֹ�����ĸ�ʴ���ڽ�������Ϳ���ᡢ��֬ | |
| D�� | ��������п��Ӧ��ȡ�������������ʵ�п�ȴ�п���������ٶȿ� |
��ʾ��Ϣ��
����������������ȫ����ʱ��ҺpHֵ���±���
| �������� | ��ȫ����pH |
| Fe2+ | 9.7 |
| Mg2+ | 12.4 |
| Fe3+ | 3.2 |
| Al3+ | 5.2 |
�ش��������⣺
��1�������ǰ����﮻�ʯҪ�����ϸ������Ŀ����������Ʒ��H2SO4�ĽӴ�������ӿ컯ѧ��Ӧ���ʣ�
��2��������У������õ���������Һ�к���Li+��SO${\;}_{4}^{2-}$��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���ʯ��ʯ���ʯ��ʯ�����Ȼ��ơ���ϡ���ᡱ���Ե�����Һ��pH��6.0��6.5�����Գ�ȥ��������Al3+��Fe3+��Ȼ�����õ�����Һ��
��3��������У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ�����ʽ���������Fe2+��Mg2+��Ca2+��
��4��������У����ɳ��������ӷ���ʽΪ2Li++CO32-=Li2CO3����

 ��֪����CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H20��CH2=CH2+Br2��BrCH2-CH2Br
��֪����CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H20��CH2=CH2+Br2��BrCH2-CH2Br���Ҵ���1��2-�������顢���ѵ��й������������±���ʾ��
| �Ҵ� | 1��2-�������� | ���� | |
| ͨ��״���µ�״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �۵�/�� | -130 | 9 | -116 |
| �е�/�� | 78.5 | 132 | 34.6 |
��1������E���������¶ȼƣ�
��2����������©�������IJ����ܵ��� ����a������ĸ����
a��ʹ©����Һ��������
b��������������������
c����ֹA��������ƿ���Һ�屬��
��3��ʵ��ʱ��A��������ƿ����뼸Ƭ���Ƭ��Ŀ���DZ�����Һ������ʱ���У����ȷ�Ӧ�����У�������ƿ���������ϩ�⣬���������ɵ��л���������Ҫ�����ѣ�
��4����Ӧ�����У���B�г��������ܣ������Һ����������˵������������D�г��ֶ������D�г��ֶ�������C�������ѳ���������ɵģ�
��5����Ӧ�����У�D��������ˮ��ȴʢ��Һ����Թܣ�����ҪĿ���Ƿ�ֹҺ��ӷ�����߷�Ӧ���ʣ�˵���Ʊ�1��2-��������ķ�Ӧ�Ѿ�������ʵ��������D��Һ�����ɫ��ȥ��D���Թ����Һ�����ɫ��
��1����ҵ�Ͽ���CO2��H2��Ӧ�ϳɼ״�����֪25�桢101kPa�£�
H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H2=-242kJ/mol-1
CH2OH��g��+$\frac{3}{2}$O2�TCO2��g��+2H2O��g�� ����H2=-676kJ/mol-1
��д��CO2��H2��Ӧ����CH2OH��H2O��g�����Ȼ�ѧ����ʽ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-50 kJ/mol��
�����б�ʾ�úϳɼ״���Ӧ�������仯ʾ��ͼ����ȷ����a������ĸ����

�ۺϳɼ״������H2�������з�Ӧ��ȡ��H2O��g��+CO��g��?H2��g��+CO2��g����ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1������ʼʱc��CO��=1mol•L-1��c��H2O��=2mol•L-1����ﵽƽ��ʱH2O��ת����Ϊ33.3%��
��2��CO��H2��ӦҲ�ܺϳɼ״���CO��g��+2H2��g��?CH3OH��g����H=-90.1kJ•mol-1����250���£���һ������CO��H2Ͷ��10L�ĺ����ܱ������У������ʵ�Ũ�ȣ�mol•L-1���仯���±���ʾ��ǰ6minû�иı���������
| 2min | 4min | 6min | 8min | �� | |
| CO | 0.07 | 0.06 | 0.06 | 0.05 | �� |
| H2 | x | 0.12 | 0.12 | 0.2 | �� |
| CH3OH | 0.03 | 0.04 | 0.04 | 0.05 | �� |
����6��8minʱֻ�ı���һ����������ı�������Ǽ���1mol��������8minʱ���÷�Ӧ�Ƿ�ﵽƽ��״̬�����ǣ���ǡ����ǡ�����
��3��CH3OH�ڴ��������¿��Ա�ֱ��������HCOOH���ڳ����£�20.00mL0.1000mol•L-1NaOH��Һ����������Ũ��HCOOH��Һ��Ϻ�������Һ�и�����Ũ�ȴ�С��ϵΪc��Na+����c��HCOO-����c��OH-����c��H+����
 25��ʱ��������ĵ��볣��Ϊ��
25��ʱ��������ĵ��볣��Ϊ��| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ��K | 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��1�����ʵ���Ũ��Ϊ0.1mol/L���������ʣ�a��Na2CO3��b��NaClO��c��CH3COONa��d��NaHCO3��pH�ɴ�С��˳���ǣ�a��b��d��c�����ţ�
��2��������0.1mol/L��CH3COOH��ˮ��Լ��1%�������룬����Һ��pH=3��������Һ������ˮϡ�ͣ���ϡ�����У����б���ʽ�����ݱ����ǣ�BD��
A��c��H+��������������B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$
C��c��H+��•c��OH-�� D��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$
��3�����Ϊ10mL pH=2�Ĵ�����Һ��һԪ��HX�ֱ������ˮϡ����1 000mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ�������ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+�����ڴ�����Һˮ���������c��H+��������ڡ��������ڡ���С�ڡ�����
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ�У�c��CH3COO-��-c��Na+��=9.9��10-7mol/L����ȷ��ֵ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ij��Һ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ��һ����I2 |
| B | ����Ʒ��Һ���ȵμӹ�����ϡ���ᣬ�ٵμ�BaCl2��Һ | �μ�ϡ���������μ�BaCl2����ְ�ɫ������ | ˵����Ʒ��Һ��һ������SO42- |
| C | ��ij��Һ�м������������ټ���ϡ���� | �а�ɫ���� | ԭ��Һ����Cl- |
| D | ��ij��Һ�м���ϡ���� | ������ɫ���� | ˵��ԭ��Һ��һ����CO32- |
| A�� | A | B�� | B | C�� | C | D�� | D |