��Ŀ����
6��̼��﮹㷺Ӧ�����մɺ�ҽҩ�������Ԧ�-﮻�ʯ����Ҫ�ɷ�ΪLi2O•Al2O3•4SiO2��Ϊԭ�����Ʊ�Li2CO3�Ĺ����������£���֪��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��
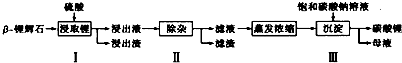
��1�������ǰ����-﮻�ʯҪ�����ϸ������Ŀ���������Һ�Ӵ�������ӿ������Ӧ���ʣ���߽�����
��2��������У������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���ʯ��ʯ���ʯ��ʯ�����������ơ���ϡ���ᡱ���Ե�����Һ��pH��6.0-6.5�����������������ӣ�Ȼ�����õ�����Һ��
��3��������У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ�����ʽ���������Fe2+��Mg2+��Ca2+��H2O2������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O
��4��������У����ɳ��������ӷ���ʽΪ2Li++CO32-=Li2CO3����
���� ��֪��-﮻�ʯ����Ҫ�ɷ�ΪLi2O•Al2O3•4SiO2��Ϊԭ�����Ʊ�Li2CO3�Ĺ������̣�Li2O•Al2O3•4SiO2�����ᣬ��ȡ�������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���ʯ��ʯ����pH��ʹAl3+��Fe3+ת��Ϊ����������������ˣ��ٽ�������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У�Fe2+������ΪFe3+��Ȼ��ת��Ϊ��������������þ����ת��Ϊ������þ������������ת��Ϊ̼��Ƴ��������˵��������Һ���ټӱ���̼������Һ�õ�̼��﮳��������˵õ�̼��ﮣ�
��1���ӷ�Ӧ���ʵĽǶ���������
��2��ץס��Ŀ�еġ�������Һ������������Һ��pH��6.0��6.5�����������Լ���ѡ�����������ҩƷ��
��3��H2O2��ǿ���������ɰ�Fe2+������Fe3+��ʯ�����̼���ƶ��dz�������ʹ��Һ�е�Fe2+��Mg2+��Ca2+������
��4������Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��֪��Li2CO3���׳�����
��� �⣺��֪��-﮻�ʯ����Ҫ�ɷ�ΪLi2O•Al2O3•4SiO2��Ϊԭ�����Ʊ�Li2CO3�Ĺ������̣�Li2O•Al2O3•4SiO2�����ᣬ��ȡ�������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���ʯ��ʯ����pH��ʹAl3+��Fe3+ת��Ϊ����������������ˣ��ٽ�������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У�Fe2+������ΪFe3+��Ȼ��ת��Ϊ��������������þ����ת��Ϊ������þ������������ת��Ϊ̼��Ƴ��������˵��������Һ���ټӱ���̼������Һ�õ�̼��﮳��������˵õ�̼��ﮣ�
��1���������ԽС����ѧ��Ӧ����Խ�죬ͬʱ��ʹ�Ԫ�ؾ����ܶ���ܽ���У�
�ʴ�Ϊ�������Һ�Ӵ�������ӿ������Ӧ���ʣ���߽����ʣ�
��2���������������У�ֻ��CaCO3�ܹ�������Һ����ȣ������Һ��pH��6.0��6.5���ɳ�ȥFe3+��Al3+��
�ʴ�Ϊ��ʯ��ʯ��
��3��������м���H2O2��Һ���ɽ�Fe2+����ΪFe3+��Ȼ�����ʯ����ɳ�ȥ���е�Fe3+��Mg2+���ټ�������Na2CO3��Һ�ɳ�ȥCa2+����ʱ��Һ�е�������Ҫ��Li+��Na+��SO42-��H2O2������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2 H+=2Fe3++2H2O��
�ʴ�Ϊ��Fe2+��Mg2+��Ca2+��2Fe2++H2O2+2 H+=2Fe3++2H2O��
��4���������뱥��Na2CO3��Һ����ҪʹLi+ת��ΪLi2CO3������
�ʴ�Ϊ��2Li++CO32-=Li2CO3����
���� �ơ���������ͭ���仯�������ճ������зdz���Ҫ�����ʣ��ر����������ڹ�����ռ�м�����Ҫ�ĵ�λ��������ص㽫���Ƽ��仯����ر���Na2O2��Na2CO3��NaHCO3�������ʡ������仯��������ԡ�Fe2+��Fe3+֮���ת����ϵ��ͭ��ұ����Cu��OH��2�����ʵȣ������Ժ�߿����⽫����ѡ�����������ӹ��桢���ӷ���ʽ��������ԭ��Ӧ�����ʵļ��������ȽǶȿ������Ԫ�ؼ��仯��������ʣ�Ҳ���ڷ�ѡ�������Խ���Ԫ�ص�����Ϊ���壬ͨ���ƶ��⡢ʵ���⣬���鿼�����ý���Ԫ�ص��й�֪ʶ��������������������ʵ��̽������ʽ����Ԫ�ػ���������ʽ���Ϊ�Ժ�߿�����������㣮

| A�� | ����10s | B�� | ����10s | C�� | С��10s | D�� | ���ж� |
| A�� | ij��ɫ��Һ�п��ܺ���SO42-��Br-��OH-��Ba2+ | |
| B�� | ʹ��ɫʯ����Һ���ɫ����Һ�п��ܺ���K+��Na+��Ba2+��HCO3- | |
| C�� | ij��Һ�У��������������ų�����Һ�п��ܺ���K+��Na+��H+��NO3- | |
| D�� | ��c��H+��=10-14mol/L����Һ�п��ܺ���Na+��AlO2-��CO32-��SO32- |
��ش��������⣺
��1��д������A�����ƣ����������ܣ�

��2��ʵ���¼���£�
| ���� | ʵ����� | ʵ������ | ʵ����� |
| �� | ����a����μ���H2SO3��Һ������ | ��ƿ����Һ�ɳȻ�ɫ��Ϊ��ɫ | Br2�������Դ���H2SO3 |
| �� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | H2O2�������Դ���Br2 |
��4��ʵ���ҳ���Cl2ͨ��NaBr��Һ���Ƶõ����壬��ҵ�ϳ��õ�ⱥ��ʳ��ˮ�Ʊ�Cl2��װ����ͼ��ʾ�����ø�װ�ã���ҵ�ϳ��õ������⣬�����Եõ�NaOH��H2���ѧʽ����װ���е����ӽ���ĤNa+����ͨ����OH-����ͨ������Ŀ���DZ���Cl2��NaOH��Ӧ����NaClO��Ӱ��NaOH�IJ����ʹ��ȣ�

����֪��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H1
N2��g��+2O2��g��=2NO2��g����H2
H2O��l��=H2O��g����H3
CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=��H1-��H2+2��H3���á�H1����H2����H3��
�����¶�ΪT1���T2��ʱ���ֱ�0.50molCH4��1.2molNO2��������̶���2L�ܱ������У������������淴Ӧ����ò�ͬʱ�̵�n��CH4���������±���
| �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1�� | n��CH4�� | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| T2�� | n��CH4�� | 0.50 | 0.30 | 0.18 | 0.15 | 0.15 |
A��T1�桢ǰ10min��V��NO2��=0.03mol/��L•min��
B��T1�桢��Ӧ�ﻯѧƽ��״̬ʱ��CH4��ת����Ϊ80%
C��T2�桢��Ӧ��40minʱ����ƽ��״̬
D��T1��T2
��2����Ӧ��ƽ�ⳣ��K��T1����K��T2������H��0���������ɱ������ݿ�֪��T2ʱ��Ӧ���ʽϴ�����T1��T2�������¶�ƽ�������ƶ�����֪K��T1����K��T2������������Ӧ���ȣ�
��3��T1��ʱ��Ӧ��ƽ�ⳣ��KΪ3.2��
��4����Ӧ��T1���½��У�50minʱ����ƽ������������ͨ��0.10molCH4��0.40molNO2������ͼ�л������£����´ﵽƽ�������n��CH4����ʱ��仯�����ߣ�ֻҪ��n��CH4���ı仯���ƣ�����Ҫȷ�����ٴ�ƽ���n��CH4����
��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ������ͼ���õ����ʹ�ù�����ʯī��缫������������Y����缫��ӦʽΪNO2-e-+NO3-=N2O5��

| A�� | 0.2 mol•L-1��ˮ�У�c��OH-����c��NH4+����� | |
| B�� | 0.1 mol/L��Na2S��Һ�У�c��OH-��=c��H+��+c��HS-��+c��H2S�� | |
| C�� | pH=3��һԪ���pH=11��һԪ��������ͺ����Һ��һ����c��OH-��=c��H+�� | |
| D�� | 10mL0.02mol•L-1HCl��Һ��10mL0.02mol•L-1Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH=12 |







