��Ŀ����
1�����ᶡ������Ҫ�Ļ���ԭ�ϣ�����ˮ����ζ��ʵ�����Ʊ����ᶡ���ķ�Ӧ��װ��ʾ��ͼ���й���Ϣ���£�CH3COOH+CH3CH2CH2CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O
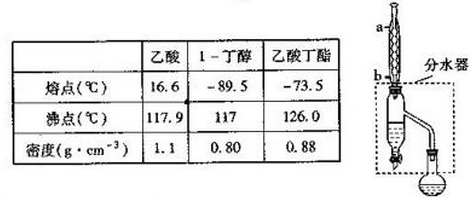
��1�����ᶡ���ֲ�Ʒ���Ʊ��ڸ����50mLԲ����ƿ�У�װ���ʯ������12.0mL��������16.0mL�����ᣨ���������ټ�3��4��Ũ���ᣮȻ���ٰ�װ��ˮ�������ã�ʵ������в��Ϸ����ȥ��Ӧ���ɵ�ˮ���������ܣ�Ȼ��С����ȣ�����ƿ�з�Ӧ��Ļ������ȴ���ˮ��������ϲ���װ������ˮӦ��b���a����b�����ܿ�ͨ�룻ͨ����ˮ���¶������ֳ�����������ˮ����Ŀ���Ƿ���������Ӧ���ɵ�ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ�
��2�����ᶡ���ľ��ƣ������ᶡ���ֲ�Ʒ�����µIJ������о��ƣ���ˮϴ������������ˮMgSO4�������10%̼����ϴ�ӣ�
����ȷ�IJ���������C�����ţ���
A���٢ڢۢ�B���ۢ٢ܢ�C���٢ܢ٢ۢ�D���ܢ٢ۢڢ�
�ڷ�Һ©�������֣���ͼ����ҺʱӦѡ��ͼ�����η�Һ©������ԭ�������η�Һ©�������������Ƚ�ϸ����˫Һ������������������ȫ��

���������ʱ�����ռ�126�����֣��е����140����л������������һ�㲻�����������ܶ��ÿ��������ܣ�����ԭ���Ƿ�ֹ���²����������ը�ѣ�
��3��������ʣ�������ˮ�����������붡����Ӧ���ɵ�ˮ���Ϊ1.8mL����������ȡ���ᶡ�������з�Ӧ���������û����ʧ���Һ��Ը���Ӧ���������ᶡ���IJ���Ϊ62.5%��
���� ��1��������ˮ���½��ϳ���ȴЧ�����жϽ�ˮ�ڣ��������ͱ����ᷴӦ�������ᶡ����ˮ��ˮ���ܶȽϴ���������ƽ�����ƣ�
��2���ٸ��ݸ����ʵ��ܽ��Լ����ʽ�������
��AΪ���η�Һ©�������ý��м�Һʹ�ã�BΪ���η�Һ©�������ڷ�Һ����ʹ�ã�
�����ᶡ���ķе���126�棬����ʱ��Ҫ�ռ��ľ������ᶡ�������������������²�̫��ᵼ���������ѣ�
��3���ȸ���������������������������ٸ���ˮ����������������ʵ����������������ֵ֮�ȼ�Ϊ���IJ��ʣ�
��� �⣺��1��������ˮ���½��ϳ���ȴЧ�����жϽ�ˮ��Ϊb���������ͱ����ᷴӦ�������ᶡ����ˮ��ˮ���ܶȽϴ�ˮ���¶������ֳ�����������ˮ���������ᶡ���ķ�ӦΪ���淴Ӧ������������Ӧ���ɵ�ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ�
�ʴ�Ϊ��b��ˮ������������Ӧ���ɵ�ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ�
��2������Ϊ�����������ᶼ���лӷ��ԣ�������ȡ�����к��������������ᣬ��һ��ˮϴ��ȥ���е�������������ڶ�����10%̼����ϴ�ӳ�ȥ�����������ˮϴ�ӳ�ȥ̼������Һ�����IJ�����ˮ����þ�����ȥˮ�����岽�����Ƶýϴ�����������ȷ��˳��٢ܢ٢ۢڣ���ѡC��
�ʴ�Ϊ��C��
��ͼ��Ϊ���η�Һ©�������ý��м�Һʹ�ã����η�Һ©�������ڷ�Һ����ʹ�ã����η�Һ©�������������Ƚ�ϸ����˫Һ������������������ȫ��Ӧѡ�����η�Һ©�����з�Һ������
�ʴ�Ϊ���棻���η�Һ©�������������Ƚ�ϸ����˫Һ������������������ȫ��
�����ᶡ���ķе���126�棬����ʱ��Ҫ�ռ��ľ������ᶡ��������Ҫ�ռ�126�����֣����������������²�̫��ᵼ���������ѣ����Էе����140����л������������һ�㲻����ˮ�������ܶ��ÿ��������ܣ�
�ʴ�Ϊ��126����ֹ���²����������ը�ѣ�
��3��������������=0.80 g/mL��12mL=9.6g�������������=1.1g/mL��16mL=17.6g��
�ȸ��ݷ���ʽ�ж��������ʹ������Բ�������Ϊ�����м��㣮
������9.6g��������ȫ��Ӧ��Ҫ�Ķ��������Ϊxg��������������Ϊzg������1.8gˮʱͬʱ��������������yg��
������������ķ�Ӧ����ʽΪCH3COOH+CH3CH2CH2CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O
60g 74g 116g 18g
9.6g xg zg
yg 1.8g
x=$\frac{9.6��74}{60}$g=11.84g��17.6g�����Զ����������������������ᶡ��������z=$\frac{116��9.6}{60}$g=18.56g��
y=$\frac{116��1.8}{18}$g=11.6g
�����������IJ���=$\frac{11.6g}{18.56g}$��100%=62.5%��
�ʴ�Ϊ��62.5%��
���� ���⿼�������ʵ��Ʊ�ʵ�鷽����ƣ���Ŀ�漰ʵ�������ʵ��ԭ�������ʵ����ʵ�Ӧ�á������ᴿ���йط���ʽ�ļ���ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�����������ݴ���������

| A�� | ������������������Ӧ Ba2++OH-+H++SO42-�TBaSO4��+H2O | |
| B�� | ����CO2ͨ��NaOH��Һ�С� CO2+OH-=H CO3- | |
| C�� | ��������Һ�м���ͭ�� Ag++Cu�TCu2++Ag�� | |
| D�� | п��ϡ���ᷴӦ Zn+2H+�TZn2++H2�� |

| A�� | �տ�ʼ��Ӧʱ���ʣ��ף��� | B�� | ƽ���Ӧ���ȣ��ף��� | ||
| C�� | 500���¸÷�Ӧƽ�ⳣ����K=3��102 | D�� | ��a��0����0.9��b��l |
 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{2}}$CH3CH2CH2CHO
��Ӧ��Ͳ��������������£�
| �е�/�� | �ܶȣ�g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.5 | 0.8107 | �� |
�ٽ�6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У�
����A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�
�۽�����ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��2����ʯ�������Ƿ�ֹ���У�
��3��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�Һʱ��ˮ���²㣨��ϡ����¡���
��4����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��5����ʵ���У�����ȩ�IJ���Ϊ51%��
��������
| A�� | 36 | B�� | 30 | C�� | 25 | D�� | 20 |
| A�� | 3 mol H2S�ɻ�ԭ4mol��KIO3 | |
| B�� | �������ͻ�ԭ�����ʵ���֮��Ϊ5��4 | |
| C�� | I2�ǻ�ԭ���K2SO4���������� | |
| D�� | 1molKIO3������0.2mol��KI |
 �Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ�Ĺ���������ʾ��
�Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ�Ĺ���������ʾ��