��Ŀ����
15�� A��B��C��D��E��F��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ��������������A��ԭ�Ӱ뾶�����ڱ�����С��Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��CԪ��ԭ�ӵ������������Ǵ�����������3����DԪ�ص�һ�����������ĵ����ֱܷ���I1=578kJ/mol��I2=1817kJ/mol��I3=2745kJ/mol��I4=11575kJ/mol��E��B���γ����������η��ӣ�F��һ��ͬλ�ص�������Ϊ63��������Ϊ34���ش��������⣺
A��B��C��D��E��F��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ��������������A��ԭ�Ӱ뾶�����ڱ�����С��Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��CԪ��ԭ�ӵ������������Ǵ�����������3����DԪ�ص�һ�����������ĵ����ֱܷ���I1=578kJ/mol��I2=1817kJ/mol��I3=2745kJ/mol��I4=11575kJ/mol��E��B���γ����������η��ӣ�F��һ��ͬλ�ص�������Ϊ63��������Ϊ34���ش��������⣺��1��A��B��C��E����Ԫ�ص縺���ɴ�С��˳��ΪO��Cl��C��H����Ԫ�ط��ű�ʾ����F�Ļ�̬ԭ����Χ�����Ų�ʽΪ3d104s1��
��2��������B2A2�����ЦҼ���м���Ŀ��Ϊ3��2��
��3����BC2��Ϊ�ȵ�����ķ�����CS2 ����N2O�����ѧʽ��д��һ�ּ��ɣ���
��4����֪����ˮDE3��178�������������DE3�ľ��������Ƿ��Ӿ��壮
��5��E��F�γɵ�һ�ֻ�����ľ�����ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��CuCl��
���� A��B��C��D��E��F��Ԫ�����ڱ���ǰ�����ڳ���Ԫ�أ�ԭ��������������A��ԭ�Ӱ뾶�����ڱ�����С����AΪH��Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��������Ų�ʽӦΪ1s22s22p2����BΪC��CԪ��ԭ�ӵ������������Ǵ�����������3������CΪO��DԪ�ص�һ�����������ĵ����ֱܷ���I1=578kJ/mol��I2=1817kJ/mol��I3=2745kJ/mol��I4=11575kJ/mol���ɼ���������ĵ����������Ʋ�DԪ���������3�����ӣ���DΪAl��E��B���γ�����������ӣ���EΪCl���γɵ�CCl4Ϊ���������η��ӣ�F��һ��ͬλ�ص�����Ϊ63��������Ϊ34����F��������Ϊ63-34=29����FΪCu�����ϣ�AΪH��BΪC��CΪO��DΪAl��EΪCl��FΪCu��
��� �⣺��1���縺��һ����������ڱ��д���������������������С����AΪH��BΪC��CΪO��EΪCl����縺��˳��ΪO��Cl��C��H��FΪCu��F�Ļ�̬ԭ����Χ�����Ų�ʽΪ3d104s1�ʴ�Ϊ��O��Cl��C��H��3d104s1��
��2��������B2A2ΪC2H2��Ϊ��Ȳ�����еĹ�����Ϊ̼̼������̼̼��������һ���Ҽ��������м������һ�������ЦҼ���ĿΪ3���м���ĿΪ2����Ϊ3��2���ʴ�Ϊ��3��2��
��3��BC2ΪCO2���ȵ�������ָԭ������ͬ�������£�ԭ�Ӽ۵�������ͬ�����ӣ�һ�㿼��Ԫ����������ƽ�Ʒ���ͬʱ��������ȷ������CO2��Ϊ�ȵ��������CS2��N2O���ʴ�Ϊ��CS2����N2O����
��4����ˮDE3ΪAlCl3����178���������¶Ƚϵͣ����жϹ���AlCl3Ϊ���Ӿ��壮�ʴ�Ϊ�����Ӿ��壮
��5��EΪCl��FΪCu�������γɵĻ�����ľ��������������������������㴦����ռ$\frac{1}{8}$�����Ĵ�����ռ$\frac{1}{2}$�������ڲ�ԭ��Ϊ�����������У���Cl�ĸ���Ϊ$8��\frac{1}{8}+6��\frac{1}{2}=4$��Cu�ĸ���Ϊ4���ɼ����γɵĻ�����Ļ�ѧʽӦΪCuCl���ʴ�Ϊ��CuCl��
���� ���⿼����Ԫ���ƶϣ������ṹ���ƶ�Ԫ�أ�ͬʱ�����˵����ܣ��縺�Ե�һ����ɣ��ȵ������֪ʶ�������ļ��㣬�����Ϊ�ۺϣ���Ŀ�ѶȲ����ǻ����⣮

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�| A�� | ��FeCl3��Һ�м���Mg��OH��2��3Mg��OH��2+2Fe3+�T2Fe��OH��3+3Mg2+ | |
| B�� | ͭ���缫��ⱥ��ʳ��ˮ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2��+2OH- | |
| C�� | �ð״׳����⣺Fe2O3+6H+�T3H2O+2Fe3+ | |
| D�� | HCO3-ˮ�⣺HCO3-+H2O?CO32-+H3O+ |
| A�� | HCl | B�� | NH4Cl | C�� | NO | D�� | C6H12O6�������ǣ� |
| A�� | 1-��ϩ�ļ���ʽ�� | B�� | ��ȩ�����ģ�ͣ� | ||
| C�� | �ǻ��ĵ���ʽ��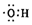 | D�� | �����ǵĽṹ��ʽ��C6H12O6 |
| A�� | ��Ȼ�� | B�� | ˮú�� | C�� | �ѽ��� | D�� | ��¯ú�� |
| A�� | ����������ˮ��Ӧ��������ת��4NA�������������Ϊ32g | |
| B�� | ��״���£�22.4L�����к���NA������ | |
| C�� | 4g��ϩ���ϩ�Ļ�����庬��0.15NA��̼ԭ�� | |
| D�� | 50mL18.4mol•L-1Ũ����������ͭ�ȷ�Ӧ������SO2������ĿΪ0.46NA |
| A�� | 25��ʱ����������Ũ�ȵ�NaOH��Һ����Һ��pHһ������7 | |
| B�� | c��C2O42- ����c�� OH-�� | |
| C�� | c��Na+��+c��H+����c��C2O42- ��+c�� HC2O4-��+c�� OH-�� | |
| D�� | c��Na+����c�� HC2O4-����c��H2C2O4����c��C2O42- �� |
| A�� | 1molCl2�μӷ�Ӧ����һ���õ�2NA������ | |
| B�� | ��״���£���22.4LNO��11.2LO2��Ϻ��Եõ�NA��NO2���� | |
| C�� | ���ڳ�ѹ�£�1L0.1mol/LHF��Һ�к���0.1NA��H+ | |
| D�� | 25�棬1.7g�ǻ�����������ΪNA |