��Ŀ����
12������200ml����KNO3��Cu��NO3��2�Ļ����Һ������c��NO3- ��=3mol•L-l����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ���2.24L���壨��״�������ٶ�������Һ�����Ϊ200ml��������˵������ȷ���ǣ�������| A�� | �������й�ת��0.4 mol���� | B�� | ԭ���Һ��c��K+��Ϊ2.0 mol•L-l | ||
| C�� | ���õ���Cu������Ϊ6.4 g | D�� | ������Һ��c��H+��Ϊ0.1 mol•L-l |
���� �������غ�����ͭ�����Һʱ�����������������ӷŵ�������������������ͭ���ӷŵ�����ͭ���ʣ���ͭ������ȫ����ʱ�������ӷŵ��������������ݵ�ʧ�����غ����ͭ�����ʵ������ٽ�ϵ���غ��������ӵ�Ũ�ȣ��ݴ˷������
��� �⣺�������غ�����ͭ�����Һʱ�����������������ӷŵ�������������������ͭ���ӷŵ�����ͭ���ʣ���ͭ������ȫ����ʱ�������ӷŵ�������������������ʵ���=$\frac{2.24L}{22.4L/mol}$=0.1mol��
ÿ����1mol����ת��4mol���ӣ�ÿ����1mol����ת��2mol���ӣ�ÿ����1molͭת��2mol���ӣ�
���Ը���ת�Ƶ����غ��ͭ�����ʵ���=$\frac{0.1mol��4-0.1mol��2}{2}$=0.1mol��
��ͭ���ӵ����ʵ���Ũ��=$\frac{0.1mol}{0.2L}$=0.5mol/L��
���ݵ���غ�ü�����Ũ��=3mol•L-1-0.5mol/L��2=2mol/L��
A��ת�Ƶ��ӵ����ʵ���=0.1mol��4=0.4mol����A��ȷ��
B�����ݷ���֪��ԭ�����Һ��c��K+��Ϊ2 mol•L-1����B��ȷ��
C���������Ϸ���֪��ͭ�����ʵ���Ϊ0.1mol������Ϊ6.4 g����C��ȷ��
D�����������ͭʱ��Һ�����������ӣ�������������Һʱ��ʵ�����ǵ��ˮ�����Ե��������ӵ����ʵ���Ϊ������4����Ϊ0.1mol��4=0.4mol����������Ũ��=$\frac{0.4mol}{0.2L}$=2mol/L����D����
��ѡ��D��
���� ���⿼���˵��ԭ������ȷ���ӷŵ�˳���ǽⱾ��ؼ������ת�Ƶ����غ㡢����غ�����������Ѷ��еȣ�

 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
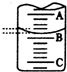
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL����ʱ�ζ�����Һ����� ������24.60ml��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
A��ʵ�����ʱ���ӵζ�����Һ�棬��ȡ�ζ��յ�ʱNaOH��Һ�����
B���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
C��ʢװ��Һ�ĵζ���װҺǰ������ˮϴ�ӣ�δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
��4�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ�����ػ���c��CH3COOH��=$\frac{c����25.35+25.30��}{2V}$mol/L��
| A�� | ���ȷ�Ӧ�ڳ����¶��������� | |
| B�� | ���ȷ�Ӧ�����ȾͲ��ᷢ�� | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ�������ȷ�Ӧ | |
| D�� | ���ݷ�Ӧ����������������������Դ�С��ȷ����Ӧ�� |
| A�� | ���� | B�� | ���ȼ� | C�� | ������ | D�� | ����� |
 ��һ���ѳ�ȥ��������Ĥ�����������������Թ���ڣ���ͼ�������Թܽ������ṯ��Һ�У�Ƭ��ȡ����Ȼ�����ڿ����У���������������������ë������īˮ���Ҷ�����������ʵ�������ж�����˵��������ǣ�������
��һ���ѳ�ȥ��������Ĥ�����������������Թ���ڣ���ͼ�������Թܽ������ṯ��Һ�У�Ƭ��ȡ����Ȼ�����ڿ����У���������������������ë������īˮ���Ҷ�����������ʵ�������ж�����˵��������ǣ�������| A�� | ʵ���з����ķ�Ӧ���ǻ��Ϸ�Ӧ | B�� | ����һ�ֽϻ��õĽ��� | ||
| C�� | ����������Ӧ�ų����������� | D�� | ��Ƭ�����ɵİ�ë�������� |
| A�� | 1L��Һ�к�142gNa2SO4 | B�� | 1L��Һ�к���1molNa+ | ||
| C�� | ��142gNa2SO4����1Lˮ�������Һ | D�� | 1Lˮ�к���1molNa2SO4 |
| A�� | 56g��������ȫ��Ӧʱʧȥ�ĵ�����Ϊһ��Ϊ2NA | |
| B�� | 18gˮ�����ĵ�����ĿΪNA | |
| C�� | �ڳ��³�ѹ�£�48g�����ͳ����Ļ������������ԭ����ĿΪ3NA | |
| D�� | �ڳ��³�ѹ�£�11.2L�������������ķ�����ĿΪ0.5 NA |
| A�� | ��һ��Ԫ����ɵ�����һ���ǵ��� | |
| B�� | �����ܵ����H+�Ļ����ﶼ���� | |
| C�� | ���ܵ����OH-��ʹ��Һ�Լ��ԣ�����Һ�Լ��ԵIJ�һ���Ǽ� | |
| D�� | ������ˮ�γɵ���Һ�ܵ��磬�����ǵ���� |
