��Ŀ����
���ۺ��������Ļ����������ȼ������ȼ��������ȷ�Ӧʱ��������ʵ��������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С�����֪��Al��Fe���۵㡢�е��������£�
��1��ijͬѧ�²⣬���ȷ�Ӧ���õ����������������Ͻ������ǣ��÷�Ӧ������ʹ���ۻ����������۵�����ͣ��������������γɺϽ�����Ϊ���Ľ����Ƿ������ ���������������������
�������������Ͻ�����������Ӳ��������
��2����������֪ʶ�ҳ�һ����֤��������Fe��������� ��
��3�����һ����ʵ�鷽����֤���������õ��������к��н�����������д����
| ���� | Al | Fe |
| �۵㣨�棩 | 660 | 1535 |
| �е㣨�棩 | 2467 | 2750 |
�������������Ͻ�����������Ӳ��������
��2����������֪ʶ�ҳ�һ����֤��������Fe���������
��3�����һ����ʵ�鷽����֤���������õ��������к��н�����������д����
| �����Լ� | NaOH��Һ |
| ʵ����������� | ȡ���� |
| �йط�Ӧ�Ļ�ѧ����ʽ | 2Al+2NaOH+2H2O��2NaAlO2+3H2�� |
���㣺̽�����ȷ�Ӧ
ר�⣺������Ҫ�Ľ������仯����
��������1�������۵�����ͣ���������Һ̬��һ�������ʢ��ɳ�ӵ��������γɺϽ𣻸��ݺϽ���������ʽ����жϣ�
��2�������ô�������������Ƿ�������
��3���������ܺ��������Ʒ�Ӧ�ų������������������������Ʋ���Ӧ���ݴ����ʵ�鷽������Ͻ��е�����
��2�������ô�������������Ƿ�������
��3���������ܺ��������Ʒ�Ӧ�ų������������������������Ʋ���Ӧ���ݴ����ʵ�鷽������Ͻ��е�����
���
�⣺��1�������۵�����ͣ���������Һ̬��һ�������ʢ��ɳ�ӵ��������γɺϽ��������ȷ�Ӧ���õ���������Ӧ�������Ͻ𣻺Ͻ��Ӳ��һ��ȸ��ɷֽ�����Ӳ�ȴ������������������Ͻ�����������Ӳ�������������Ͻ�
�ʴ�Ϊ�������������Ͻ�
��2����֤��������Fe����ķ���Ϊ���ô������������������֤��������Fe��
�ʴ�Ϊ���ô������������������֤��������Fe��
��3���������ܺ��������Ʒ�Ӧ�ų�������2Al+2OH-+2H2O=2AlO2-+3H2���������������������Ʋ���Ӧ������������������Һ֤���������õĿ�״�������к��н���������������Ϊ��ȡ����������μ�����������Һ���������ݲ�����֤�����������н�������
�ʴ�Ϊ�������NaOH��Һ��
�ʴ�Ϊ�������������Ͻ�
��2����֤��������Fe����ķ���Ϊ���ô������������������֤��������Fe��
�ʴ�Ϊ���ô������������������֤��������Fe��
��3���������ܺ��������Ʒ�Ӧ�ų�������2Al+2OH-+2H2O=2AlO2-+3H2���������������������Ʋ���Ӧ������������������Һ֤���������õĿ�״�������к��н���������������Ϊ��ȡ����������μ�����������Һ���������ݲ�����֤�����������н�������
�ʴ�Ϊ�������NaOH��Һ��
���������⿼�����ȷ�Ӧԭ�����������Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ�ע���������ȷ�Ӧ������������ȷ���Ļ�ѧ���ʼ��Ͻ���������ʣ���1��Ϊ�״��㣬ע������������ݣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���ڷ�ӦaA+bB=dD+eE���û�ѧ��Ӧ���ʶ���Ϊv=
=
=
=
��ʽ��v��X��ָ����X=��X=A��B��D��E���ķ�Ӧ���ʣ�a��b��d��e�ǻ�ѧ��������298kʱ�������Һ�еķ�ӦH2O2+2HI�T2H2O+I2�ڲ�ͬŨ��ʱ��ѧ��Ӧ����v���±�������˵����ȷ���ǣ�������
| v(A) |
| a |
| v(B) |
| b |
| v(D) |
| d |
| v(E) |
| e |
| ʵ���� | 1 | 2 | 3 | 4 |
| c��HI��/mol?L-1 | 0.100 | 0.200 | 0.300 | 0.100 |
| c��H2O2��/mol?L-1 | 0.100 | 0.100 | 0.100 | 0.200 |
| v/mol?L-1?s-1 | 0.00760 | 0.0153 | 0.0227 | 0.0151 |
| A��ʵ��1��2�У�v��H2O2����� |
| B����Ũ�Ⱦ�Ϊ0.200mol?L-1 H2O2��HI��Һ�������ϣ���Ӧ��ʼʱv=0.0304mol?L-1?s-1 |
| C��v�롰HI��H2O2Ũ�ȵij˻����ı�ֵΪ���� |
| D��ʵ��4����Ӧ5���H2O2Ũ�ȼ�����0.0755mol?L-1 |
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ�ǣ�������
| A��������������Һ�м����������ۣ�2Al+2OH-+2H2O�T2AlO2-+3H2�� |
| B��NH4HCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-�TBaCO3��+H2O |
| C��������Һ�Լ��Ե�ԭ��S2-+H2O�TH2S+2OH- |
| D����FeBr2��Һ��ͨ������������2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- |
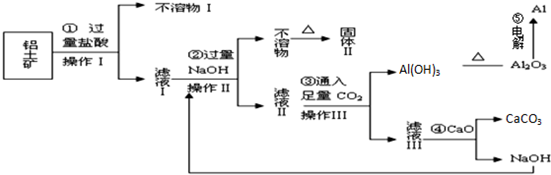
 �±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�
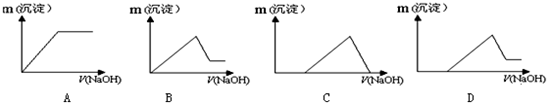
�±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�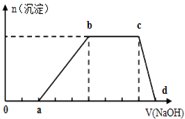 ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��Cu2+��SO42-��I-��CO32-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ�����ֳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪��
ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��Cu2+��SO42-��I-��CO32-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ�����ֳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪��