��Ŀ����
17��SO2�ĺ����ǿ��������ձ���һ����Ҫ���ָ�꣬Ҳ���������������Ű�ҹ��ֵ�������Ҫԭ��֮һ���Ӵ�SO2�Ĵ������ȣ�������������Ⱦ�ĵ���֮����
�绯ѧ������SO2�����Ṥҵβ���е�SO2������������Ʊ����ᣬͬʱ��õ��ܣ�װ����ͼ1��ʾ���缫��Ϊ���Բ��ϣ���

��1��M�������ĵ缫��ӦʽΪSO2-2e-+2H2O=4H++SO42-��
��2����ʹ��װ�õĵ���ǿ�ȴﵽ2.0A��������ÿ����Ӧ��ͨ���״������������Ϊ0.014L����֪��1��e��������Ϊ1.6��10-19C����
����Һ��绯ѧ�ۺϣ��Ƽ�ѭ����������SO2��
��3���Ƽ�ѭ�����У���Na2SO3��Һ��Ϊ����Һ������SO2���÷�Ӧ�����ӷ���ʽΪSO32-+SO2+H2O=2HSO3-��
��4������Һ����SO2�Ĺ����У�pH��n��SO32-��/n��HSO3-���仯��ϵ��ͼ2��ʾ��
����ͼ�����ݺͱ仯����˵��NaHSO3��Һ�����Ե�ԭ����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$�ļ�С��PH��С��PH��С����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��10��PH=6.2����Һ�������ԣ���ΪNaHSO3��Һ����ҺPHһ��С��6.2����Һ�����ԣ�
��n��SO32-��/n��HSO3-��=1��1ʱ����Һ������Ũ���ɴ�С��˳����c��Na+����c��SO32-��=c��HSO3-����c��OH-����c��H+����
��5��������Һ��pH����ԼΪ6ʱ�����������۴�����ֱ���õ�pH��8������Һ��ѭ�����ã�����ʾ��ͼ��ͼ3��
��д�����������ĵ缫��ӦʽHSO3-+H2O-2e-=SO42-+3H+��
�ڵ��缫����2mol����ת��ʱ�������������Ϊ2g��
���� ��1����װ����ԭ��أ���Ӧԭ��Ϊ��������������ˮ��Ӧ�������ᣬ�����ϵ�����ʧ���ӷ���������Ӧ�������ϵ����ʵõ��ӷ�����ԭ��Ӧ���ݴ˷���M�������ĵ缫��Ӧʽ��
��2������Q=It��N=$\frac{Q}{e}$���SO2-2e-+2H2O=4H++SO42-���㣻
��3��SO2��Na2SO3��Һ�����������������ƣ�����������ǿ�������ȫ���룻
��4����NaHSO3��Һ����Ҫ��HSO3-���ڣ�HSO3-�ĵ������������n��SO32-����n��HSO3-����1��1������ͼ��֪����������������ӵ����ʵ�����������������ӵ����ʵ���ʱ��������������Һ�����ԣ�������������Ӽ���ˮ�����ܵ��룬������������Һ������ͬʱ˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$�ļ�С��PH��С��PH��С����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��10��PH=6.2����Һ�������ԣ���ΪNaHSO3��Һ����ҺPHһ��С��6.2��
��$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$��=1��1����ҺΪNaHSO3��Һ��Na2SO3��Һ����ҺPH=7.2����Һ���Լ��ԣ�˵��HSO3-�ĵ���̶�С��ˮ��̶ȣ�
��5���������������ӷŵ緢��������Ӧ��
��������Ϊ������H+�õ�������������
��� �⣺��1����װ����ԭ��أ���Ӧԭ��Ϊ��������������ˮ��Ӧ�������ᣬͨ��������N�缫��������ԭ��طŵ�ʱ���������ɸ������������������������õ��Ӻ������ӷ�Ӧ����ˮ���缫��ӦʽΪ��O2+4e-+4H+=2H2O������M�ϣ���������ʧ���Ӻ�ˮ��Ӧ�������ᣬ�缫��ӦʽΪ��SO2-2e-+2H2O=4H++SO42-��
�ʴ�Ϊ��SO2-2e-+2H2O=4H++SO42-��
��2������Q=It=2A��60s=120C��N=$\frac{Q}{e}$=$\frac{120}{1.6��1{0}^{-19}}$=7.5��1020����
��SO2��������2e-
22.4 2��6.02��1023
V 7.5��1020
V=$\frac{22.4��7.5��1{0}^{20}}{2��6.02��1{0}^{23}}$��0.014L��
�ʴ�Ϊ��0.014��
��3��SO2��Na2SO3��Һ�����������������ƣ����ӷ�ӦΪSO32-+SO2+H2O=2HSO3-��
�ʴ�Ϊ��SO32-+SO2+H2O=2HSO3-��
��4����ͼ�������֪��$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��1����ҺPH=7.2��$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$��1��1��PH��7����Һ�����ԣ�$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��10��PH=6.2����Һ�����ԣ����Ե�ΪNaHSO3��Һ��NaHSO3��Һ��HSO3-���ڵ���ƽ�⣬HSO3-?H++SO32- ��ˮ��ƽ�⣺HSO3-+H2O?H2SO3+OH-��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ���$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$�ļ�С��PH��С��PH��С����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��10��PH=6.2����Һ�������ԣ���ΪNaHSO3��Һ����ҺPHһ��С��6.2����Һ�����ԣ�
�ʴ�Ϊ����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$�ļ�С��PH��С��PH��С����$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��10��PH=6.2����Һ�������ԣ���ΪNaHSO3��Һ����ҺPHһ��С��6.2����Һ�����ԣ�
��$\frac{n��S{{O}_{3}}^{2-}��}{n��HS{{O}_{3}}^{-}��}$=1��1����ҺΪNaHSO3��Һ��Na2SO3��Һ����ҺPH=7.2����Һ���Լ��ԣ�˵��HSO3-�ĵ���̶�С��ˮ��̶ȣ���Һ������Ũ�ȴ�СΪ��c��Na+����c��SO32-��=c��HSO3-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��SO32-��=c��HSO3-����c��OH-����c��H+����
��5���ٵ�����Һ��pH����ԼΪ6ʱ������Һ����������Ҫ��������������ӣ����������������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ��缫��ӦʽΪ��HSO3-+H2O-2e-=SO42-+3H+��
�ʴ�Ϊ��HSO3-+H2O-2e-=SO42-+3H+��
��������Ϊ������H+�õ���������������缫��ӦʽΪ��2H++2e-=H2�������Ե��缫����2mol����ת��ʱ������1mol���������������������Ϊ2g��
�ʴ�Ϊ��2g��
���� �����ۺ��Խϴ��漰�����ӷ���ʽ�����ԭ��������Ũ�ȴ�С�ıȽϡ���ѧƽ��Ӱ�������Լ��绯ѧ֪ʶ��֪ʶ�㣬Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬�ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���ȶ��ԣ�Na2CO3��NaHCO3 | |
| B�� | ��ͬ�¶�����ˮ�е��ܽ�ȣ�Na2CO3��NaHCO3 | |
| C�� | ȡ1 mol•L-1��������Һ���ֱ�μӳ���ʯ��ˮ���г������ɵ���Na2CO3��Һ | |
| D�� | ȡ�����������ֹ���ֱ����Ũ�ȵ���������ᷴӦ���������ݽϿ����NaHCO3��Һ |
| A�� | X��Y�γɵĻ������У�X�����Ը��ۣ�Y������ | |
| B�� | ��ۺ���������ԣ�X��Ӧ������ǿ��Y��Ӧ������ | |
| C�� | ��һ������Xһ������Y | |
| D�� | ��̬�⻯����ȶ��ԣ�HmYС��HnX |
��SO2������ʹ��ˮ��ɫ
��SO2��ʹ���Ը��������Һ��ɫ
��SO2������ʹƷ����Һ��ɫ
��SO2������ʹ��ɫ�IJ�ñ���ɫ��
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
 ��
�� ���ֿռ���ʽ��ͬ���칹�壩���ж����л������о����������ֿռ�������ʽ���ǣ�������
���ֿռ���ʽ��ͬ���칹�壩���ж����л������о����������ֿռ�������ʽ���ǣ�������| A�� | ��ϩ | B�� | 2-��ϩ | C�� | ����ϩ | D�� | ���� |
| A�� | 14 | B�� | 15 | C�� | 16 | D�� | 17 |
| A�� | Ԫ��X�γɵĵ���һ����ԭ�Ӿ��� | |
| B�� | X��Z�γɵĻ���������۵�ߡ�Ӳ�ȴ���ص� | |
| C�� | Y����Ԫ���γɵĻ�����Y2O2�����������ӵĸ�����Ϊ1��1 | |
| D�� | W�ֱ���Y��Z�γɵĻ������к��еĻ�ѧ��������ͬ |
 ��֪X�ĵ縺����ͬ����Ԫ����������̬ԭ��������Ӳ��p����ʰ����״̬���ش��������⣺
��֪X�ĵ縺����ͬ����Ԫ����������̬ԭ��������Ӳ��p����ʰ����״̬���ش��������⣺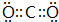 ��B��D�γɵ�ԭ�Ӹ�����Ϊl��l�Ļ������к��еĻ�ѧ�����������Ӽ����ۼ���
��B��D�γɵ�ԭ�Ӹ�����Ϊl��l�Ļ������к��еĻ�ѧ�����������Ӽ����ۼ���