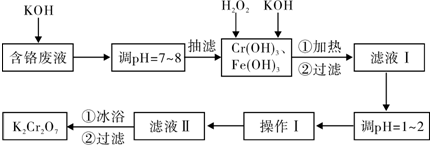
��Ŀ����
���ڻ����ķ�����ᴿ�����õķ����У����ˡ�������������ȡ���ᾧ�����ȷֽ�ȣ����и�������ķ�����ᴿӦ����ʲô������
��1��ʵ�����е�ʯ��ˮ���ã�Һ���ϳ�������CaCO3��Ĥ������ ������ȥ��
��2���ᴿ��������KCl��KNO3ʱ���ɲ��� ������
��3����ȡ��ˮ�е��壬�� �ķ�����
��4����ȥʳ����Һ�е�ˮ���ɲ��� ������
��5��������ˮ���ɲ��� ������
��1��ʵ�����е�ʯ��ˮ���ã�Һ���ϳ�������CaCO3��Ĥ������
��2���ᴿ��������KCl��KNO3ʱ���ɲ���
��3����ȡ��ˮ�е��壬��
��4����ȥʳ����Һ�е�ˮ���ɲ���
��5��������ˮ���ɲ���
���㣺���ʷ��롢�ᴿ��ʵ�鷽�����,���ʵķ��롢�ᴿ�Ļ�������ѡ����Ӧ��
ר�⣺ʵ����
��������1��̼��Ʋ�����ˮ����������������ˮ��
��2��KNO3���ܽ�����¶�Ӱ���KCl���ܽ�����¶�Ӱ��С��
��3���岻������ˮ�����������Ȼ�̼��
��4��ʳ��ˮ�У�NaCl����ˮ��
��5��������ˮ������ˮ���������ʵķе����ϴ���룮
��2��KNO3���ܽ�����¶�Ӱ���KCl���ܽ�����¶�Ӱ��С��
��3���岻������ˮ�����������Ȼ�̼��
��4��ʳ��ˮ�У�NaCl����ˮ��
��5��������ˮ������ˮ���������ʵķе����ϴ���룮
���
�⣺��1��̼��Ʋ�����ˮ����������������ˮ����ʯ��ˮ���ã�Һ���ϳ�������CaCO3��Ĥ�����ù��˷�����ȥ���ʴ�Ϊ�����ˣ�
��2��KNO3���ܽ�����¶�Ӱ���KCl���ܽ�����¶�Ӱ��С�����ᴿ��������KCl��KNO3ʱ���ɲ��ýᾧ�������ʴ�Ϊ���ᾧ��
��3���岻������ˮ�����������Ȼ�̼����ȡ��ˮ�е��壬����ȡ�ķ������ʴ�Ϊ����ȡ��
��4��ʳ��ˮ�У�NaCl����ˮ�����ȥʳ����Һ�е�ˮ���ɲ��������������ʴ�Ϊ��������
��5��������ˮ������ˮ���������ʵķе����ϴ���룬����ˮ���ɲ����������ʴ�Ϊ������
��2��KNO3���ܽ�����¶�Ӱ���KCl���ܽ�����¶�Ӱ��С�����ᴿ��������KCl��KNO3ʱ���ɲ��ýᾧ�������ʴ�Ϊ���ᾧ��
��3���岻������ˮ�����������Ȼ�̼����ȡ��ˮ�е��壬����ȡ�ķ������ʴ�Ϊ����ȡ��
��4��ʳ��ˮ�У�NaCl����ˮ�����ȥʳ����Һ�е�ˮ���ɲ��������������ʴ�Ϊ��������
��5��������ˮ������ˮ���������ʵķе����ϴ���룬����ˮ���ɲ����������ʴ�Ϊ������
���������⿼�����ʷ��롢�ᴿʵ�鷽������ƣ�Ϊ��Ƶ���㣬�������ʵ����ʼ�����������ʵIJ�����Ʒ��뷽��Ϊ���Ĺؼ���ע�ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���й���ʵ���������������ǣ�������
| A������NaOH��Һʱ���ó���ֽ��ȡNaOH��������ҺŨ��ƫ�� |
| B���ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һʱ����ƿ��NaOH��Һ��ϴ�����ý��ƫ�� |
| C���ⶨ����ͭ�����нᾧˮ�����Ĺ����У���С�ļ�����������ڣ����ý��ƫ�� |
| D����������Ħ�����ʱ��þ������û�д��������ý��ƫ�� |
����������ͭ���Ȼ�ͭ��Һ�����ᡢ����������ҺΪ��Ӧ������䷢���ķ�Ӧ�У���֪Fe��CuO�ڸ������ܷ�Ӧ����������
| A��6�� | B��5�� | C��4�� | D��3�� |