��Ŀ����
��ѧ��ʵ�鷽��Ϊ����̽�����������ṩ��һ�ѽ�Կ�ף�����ʵ�鷽����ȷ���ǣ�������
| A��ʵ���ҿ�ͨ������ķ������Ӻ�Fe3+������ˮ�л����������ˮ |
| B������Ʒ�м��������ữ���Ȼ�����Һ����ȷ����Ʒ���Ƿ���SO42- |
| C���ýྻ�IJ�����պȡ����Һ��Ʒ��Һ���ھƾ��ƻ��������գ�����Ƿ���Na+ |
| D���ø����ҽྻ�IJ�����պȡ������Һ����ʪ���pH��ֽ�в���������ɫ�����տɲⶨ��Һ��pHֵ |
���㣺��ѧʵ�鷽��������,���ʵļ���ͼ���Ļ�������ѡ��Ӧ��
ר�⣺���ʼ��������
������A����Fe3+������ˮ��ˮ�ķе�ͣ�
B��������������ӣ�Ӧ�ȼ������ų��������ӵĸ��ţ�
C���������к���Ԫ�أ�
D���ⶨpH��ֽ����ֽ����ʪ��
B��������������ӣ�Ӧ�ȼ������ų��������ӵĸ��ţ�
C���������к���Ԫ�أ�
D���ⶨpH��ֽ����ֽ����ʪ��
���
�⣺A����Fe3+������ˮ��ˮ�ķе�ͣ�����������ķ����Ӻ�Fe3+������ˮ�л����������ˮ����A��ȷ��
B��������������ӣ�Ӧ�ȼ������ų��������ӵĸ��ţ��������ټ��Ȼ����������ɳ���������������ӣ���B����
C���������к���Ԫ�أ�Ӧ���ò�˿պȡ����Һ��Ʒ��Һ���ھƾ��ƻ��������գ�����Ƿ���Na+����C����
D���ⶨpH��ֽ����ֽ����ʪ�����ø����ҽྻ�IJ�����պȡ������Һ���ڸ����pH��ֽ�в���������ɫ�����տɲⶨ��Һ��pHֵ����D����
��ѡA��
B��������������ӣ�Ӧ�ȼ������ų��������ӵĸ��ţ��������ټ��Ȼ����������ɳ���������������ӣ���B����
C���������к���Ԫ�أ�Ӧ���ò�˿պȡ����Һ��Ʒ��Һ���ھƾ��ƻ��������գ�����Ƿ���Na+����C����
D���ⶨpH��ֽ����ֽ����ʪ�����ø����ҽྻ�IJ�����պȡ������Һ���ڸ����pH��ֽ�в���������ɫ�����տɲⶨ��Һ��pHֵ����D����
��ѡA��
���������⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰���������ᴿ���������Ӽ��顢pH�IJⶨ�ȣ�����ʵ��������ܵĿ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
�����Ŀ
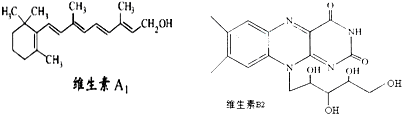
ά����A1��B2�Ľṹ��ʽ�ֱ���ͼ��ʾ����֪��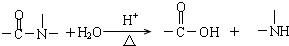 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������
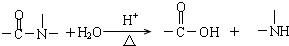 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������
| A��1molά����A1�������ˮ�е�4molBr2�����ӳɷ�Ӧ |
| B��ά����A1�ķ���ʽΪC19H30O����һ��������ˮ�ĸ߷��� |
| C��ά����B2�����������³���ˮ���õ����л��������ڷ��������ö����� |
| D����-C4H9ȡ��ά����B2�����ϵ�һ��Hԭ�ӣ����ɵ�4��ͬ���칹�� |
�����й�˵������ȷ���ǣ�������
| A�������£�PbSO4������pH=7��CH3COONH4��Һ��˵����CH3COO��2Pb��������� |
| B����Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ�������Ksp��BaCO3����Ksp��BaSO4�� |
| C��DZͧ�ϵĺ˷�Ӧ��ʹ��Һ̬��-�ƺϽ������Ƚ��ʣ����Ͻ���n��Na����n��Al��������Ͷ�뵽������ˮ�пɵ���ɫ����Һ |
| D������֧ʢ��KI3��Һ���Թ��У��ֱ�μӵ�����Һ��AgNO3��Һ��ǰ����Һ�����������л�ɫ������˵��KI3��Һ�д���ƽ�⣺I3-�TI2+I- |
��ҵ���ú�п���ϣ���FeO��CuO�����ʣ���ȡ����ZnO���������£�

����֪Ksp[Fe��OH��3]=2.6��10-39��Ksp[Cu��OH��2]=2.2��10-20��������˵������ȷ���ǣ�������

����֪Ksp[Fe��OH��3]=2.6��10-39��Ksp[Cu��OH��2]=2.2��10-20��������˵������ȷ���ǣ�������
| A�����������У�����ʱ�õ���60% H2SO4���ܶ���1.5 g/cm3����������100 mL����H2SO4��Һ������Ҫ18.4 mol?L-1��Ũ����ԼΪ49.9mL |
| B������������H2O2��ֻ��Fe��OH��3�������֣�����Һ��c��Fe3+��=2.6��10-15mol?L-1������Һ��c��Cu2+����2.2��10-4mol?L-1 |
| C��������NH4HCO3�����ɵij�����Zn5��OH��6��CO3��2����÷�ӦΪ5ZnSO4+10NH4HCO3=Zn5��OH��6��CO3��2��+5��NH4��2SO4+8CO2��+2H2O |
| D�������ɵij�������̬��ΪZna��OH��b ��CO3��c�ģ�a��b��c���������������ּ�ʽ̼��п�Ļ�����ֱ������Zn5��OH��6��CO3��2 ��Zn3��OH��6CO3 |
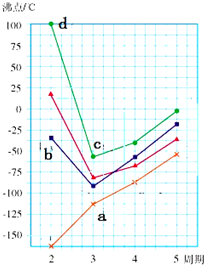 A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�أ�A��B���γ�B2A��B2A2���ֻ����B��D��G������������Ӧˮ��������֮�䶼�ܷ�Ӧ��D��F��Gԭ������������֮�͵���15��
A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�أ�A��B���γ�B2A��B2A2���ֻ����B��D��G������������Ӧˮ��������֮�䶼�ܷ�Ӧ��D��F��Gԭ������������֮�͵���15��