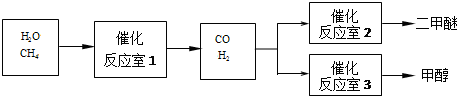
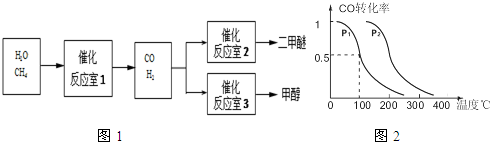
��Ŀ����
��NAԼΪ6.02��1023��������ȷ�𰸲���գ�
��1��3.01��1023��OH-�����ʵ���Ϊ mol�����е��ӵ����ʵ���Ϊ mol��
��2����16g���������з�������ͬ�İ����� L����״���£����ð���������ԭ���������ʵ����� mol���ð�����������������Լ ����
��3��5Lij��������Һ�к���9.03��1022��Fe3+�������Һ��SO42-�����ʵ����� mol������Һ��������������Ϊ g�������������ʵ���Ũ���� mol/L������֪��Fe2��SO4��3����Һ�з������� Fe2��SO4��3�T2Fe3++3SO42- ��
��1��3.01��1023��OH-�����ʵ���Ϊ
��2����16g���������з�������ͬ�İ�����
��3��5Lij��������Һ�к���9.03��1022��Fe3+�������Һ��SO42-�����ʵ�����
���㣺���ʵ�������ؼ���,�����ӵ�����
ר�⣺�����ӵ������Ͱ����ӵ�����
��������1������n=
��������������ӵ����ʵ������ǻ��к���10�����ӣ��������������ӵ����ʵ�����������еĵ��ӵ����ʵ�����
��2������n=
��V=n?Vm�����16g�����ڱ���µ��������������ͬ������������Ͱ����������ͬ�����ݰ��������ʵ�����������е�ԭ�ӵ����ʵ���������N=nNA����������������ӵ���Ŀ��
��3������n=
����������ӵ����ʵ������ٸ����������Ļ�ѧʽ�������������ӵ����ʵ����������������ʵ������ټ���������������������ʵ���Ũ�ȣ�
| N |
| NA |
��2������n=
| m |
| M |
��3������n=
| N |
| NA |
���
�⣺��1��3.01��1023��OH-�����ʵ���Ϊ��
=0.5mol��0.5mol���������Ӻ���5mol���ӣ�
�ʴ�Ϊ��0.5��5��
��2��16g���������ʵ���Ϊ��
=0.5mol�������������ķ�������ͬ�����������ʵ���Ϊ0.5mol�������0.5mol���������Ϊ��22.4L/mol��0.5mol=11L��0.5mol�����к��е�ԭ�ӵ����ʵ���Ϊ��0.5mol��4=2mol��0.5mol�������еķ�����Ϊ��6.02��1023��0.5=3.01��1023��
�ʴ�Ϊ��11.2��2��3.01��1023��
��3��9.03��1022��Fe3+�����ʵ���Ϊ��
=0.15mol������Fe2��SO4��3��֪�������������ʵ���Ϊ��0.15mol��
=0.075mol��0.075mol�������к�����������ӵ����ʵ���Ϊ��0.075mol��3=0.225mol��0.075mol������������Ϊ��400g/mol��0.075mol=30g�������������ʵ���Ũ��Ϊ��
=0.015mol/L��
�ʴ�Ϊ��0.225��30��0.015��
| 3.01��1023 |
| 6.02��1023 |
�ʴ�Ϊ��0.5��5��
��2��16g���������ʵ���Ϊ��
| 16g |
| 32g/mol |
�ʴ�Ϊ��11.2��2��3.01��1023��
��3��9.03��1022��Fe3+�����ʵ���Ϊ��
| 9.03��1022 |
| 6.02��1023 |
| 1 |
| 2 |
| 0.075mol |
| 5L |
�ʴ�Ϊ��0.225��30��0.015��
���������⿼�����й����ʵ����ļ��㣬��Ŀ�Ѷ��еȣ�ע���������ʵ����밢��٤��������Ħ������������Ħ�������������֮���ת����ϵ����ȷ�й����ʵ����ļ��㷽����

��ϰ��ϵ�д�
�����Ŀ
������ͼװ�ã�����ɺܶ�绯ѧʵ�飮�����йش�װ�õ������У�������ǣ�������


| A����XΪп����YΪNaCl��Һ������K����M�����ɼ������ĸ�ʴ�����ַ�����Ϊ������������������ |
| B����XΪ̼����YΪNaCl��Һ������K����N�����ɼ������ĸ�ʴ�����ַ�����Ϊ��ӵ������������� |
| C����XΪͭ����YΪ����ͭ��Һ������K����M��ʱ�����缫�ϵĵ缫��ӦʽΪCu2++2e��Cu |
| D����XΪͭ����YΪ����ͭ��Һ������K����N��ʱ����Һ�и�����Ũ�ȶ����ᷢ���仯 |
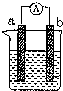 ��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺
��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺