��Ŀ����
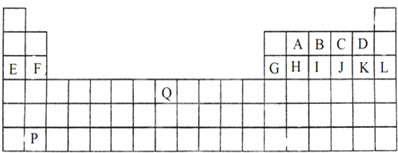
1������Ԫ�����ڱ���һ���֣���ش��й����⣺| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
��2���ڢۢ�����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����Na��Mg��F ����Ԫ�ط��ű�ʾ��
��3���õ���ʽ��ʾ������γɻ�����Ĺ���
 ��
����4���ڢ١���Ԫ���У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4��������ǿ����NaOH�����ѧʽ��
��5���ڢ����ĵ����У���ѧ���ʽϻ��õ���Cl2���ѧʽ��������ʲô��ѧ��Ӧ˵����д����Ӧ�Ļ�ѧ����ʽ����Cl2+2Br-=2Cl-+Br2��
���� ��Ԫ�������ڱ���λ�ã���֪��ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
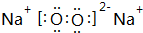
��3��������γɻ�����ΪNaCl�����������������ӹ��ɣ�����ԭ�ӡ���ԭ�ӵ���ʽ��ʾ���γɹ��̣�
��4���ڢ١���Ԫ���У�Ԫ�ص�����������Ӧ��ˮ������������ǿ���Ǹ����ᣬNa�Ľ�������ǿ���������Ƶļ�����ǿ��
��5���ǽ�����Խǿ������Խ���ã����Ը��ݵ���֮����û�����֤����
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��Ar�Ļ�ѧ��������ã��ʴ�Ϊ��Ar��
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶Na��Mg��F���ʴ�Ϊ��Na��Mg��F��
��3��������γɻ�����ΪNaCl�����������������ӹ��ɣ��õ���ʽ��ʾ���γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��4���ڢ١���Ԫ���У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4��Na�Ľ�������ǿ���������Ƶļ�����ǿ���ʴ�Ϊ��HClO4��NaOH��
��5���ǽ�����Cl��Br����Cl2�����ã����Ը��ݵ���֮����û�����֤������Ӧ���ӷ���ʽΪ��Cl2+2Br-=2Cl-+Br2��
�ʴ�Ϊ��Cl2��Cl2+2Br-=2Cl-+Br2��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ����ض�Ԫ�������ɵĿ��飬ע�����ս����ԡ��ǽ�����ǿ���Ƚϼ�ʵ����ʵ��

| A�� | ɳ�Ӻ�ճ����Ҫ�ɷ�Ϊ������ | |
| B�� | ��ȼн�ٻ�ʹճ���������ӵ�������ѧ�仯 | |
| C�� | ���ƺ���Ȼ��ȴ�ɺ��ߣ���ˮ��ȴ������ | |
| D�� | ճ��������ש�ߺ��մɵ���Ҫԭ�� |
| A�� | 7 | B�� | 8 | C�� | 9 | D�� | 10 |
| A�� | ���µIJ��� | B�� | �뵼�� | C�� | ���� | D�� | ũҩ |
| A�� | ԭ�������Һ�ܵ���������ƶ������� | |
| B�� | �缫�Ͻ��е�������ԭ��Ӧ��������Ϊ���� | |
| C�� | ���������߽����������Բ�ͬ�ĵ缫���� | |
| D�� | �缫�Ͻ��е�������ԭ��Ӧ�з����˵��ӵ�ת�� |
| A�� | ���ԣ�H2SO4��H3PO4 | B�� | �ǽ����ԣ�Cl��Br | ||
| C�� | ԭ�Ӱ뾶��S��O��F | D�� | ���ȶ��ԣ�Na2CO3��NaHCO3 |
 ��
��