��Ŀ����
9��ͼ����ѧ�̲���Ԫ�����ڱ���һ���֣����б��A��Q14��Ԫ�أ��Իش��������⣺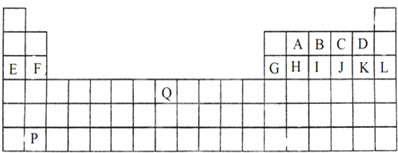
��1�����ϱ����г��Ķ�����Ԫ���У���������ʻ�ѧʽ��
ԭ�Ӱ뾶��С���ǣ���ϡ������Ԫ�أ�F�������ӻ�ԭ����������F-������������Ӧ��ˮ������������ǿ����HClO4��
��2��F��G��Ԫ����Ƚϣ������Խ�ǿ����þ�������ƣ���������֤�ý��۵�ʵ����bc�������ţ�
a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
b����������Ԫ�صĵ��ʷ�ĩ�ֱ��ͬŨ�ȵ����ᷴӦ
c����������Ԫ�صĵ��ʷ�ĩ�ֱ����ˮ���ã��������̪��Һ
d���Ƚ�������Ԫ�ص���̬�⻯����ȶ���
��3��BԪ���γɵĵ��ʵĽṹʽΪN��N��E2C2�ĵ���ʽΪ
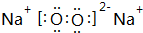 ��
����4��д��E������������Ӧ��ˮ������G���ʷ����ķ�Ӧ�����ӷ���ʽ��2Al+2H2O+2OH-=2AlO2-+3H2����
���� ��Ԫ�������ڱ��е�λ�ÿ�֪��AΪ̼��BΪ����CΪ����DΪ����EΪNa��FΪMg��GΪAl��HΪSi��IΪ�ס�JΪ��KΪCl��LΪAr��PΪBa��QΪFe��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ͬ����������ҷǽ�������ǿ��ͬ�������϶��·ǽ����Լ������ǽ�����Խǿ�������ӻ�ԭ��Խ��������������Ӧ��ˮ������������ǿ���Ǹ����
��2��ͬ����������ҽ����Լ�����������ˮ���ᷴӦ�����ס����ҳ̶ȣ���������������Ӧˮ����ļ���ǿ�����жϽ�����ǿ����
��3��BԪ���γɵĵ���ΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ�Na2O2������������������ӹ��ɣ�
��4��E������������Ӧ��ˮ����ΪNaOH��Al���������Ʒ�Ӧ��Ӧ����ƫ��������������
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪��AΪ̼��BΪ����CΪ����DΪ����EΪNa��FΪMg��GΪAl��HΪSi��IΪ�ס�JΪ��KΪCl��LΪAr��PΪBa��QΪFe��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶��������Ԫ����Fԭ�Ӱ뾶��С��FԪ�طǽ�����Խǿ����F-���ӻ�ԭ������������������Ӧ��ˮ������������ǿ����HClO4��
�ʴ�Ϊ��F��F-��HClO4��
��2��ͬ����������ҽ����Լ�������Mg�Ľ����Ա�Al��ǿ��
a��Mg��Al����Ԫ���ڿ����о��ã�����������������Ĥ���ֱ������ˮ�У����ܸ��ݷ�Ӧ�жϽ�����ǿ������a����
b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ����ӦԽ���ң�˵��������Խǿ����b��ȷ��
c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ����Һ����Խǿ��˵��������Խǿ����c��ȷ��
d�����߶��ǽ�����û����̬�⻯����ܱȽ��⻯����ȶ����жϽ�����ǿ������d����
�ʴ�Ϊ��þ��bc��
��3��BԪ���γɵĵ���ΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ��ṹʽΪN��N��Na2O2������������������ӹ��ɣ�����ʽΪ ��
��
�ʴ�Ϊ��N��N�� ��
��
��4��E������������Ӧ��ˮ����ΪNaOH��Al���������Ʒ�Ӧ��Ӧ����ƫ����������������Ӧ���ӷ���ʽΪ��2Al+2H2O+2OH-=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2H2O+2OH-=2AlO2-+3H2����
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ����ض�Ԫ���������뻯ѧ����飬���������Ԫ�����ڱ������ս����ԡ��ǽ�����ǿ���Ƚ�ʵ����ʵ��

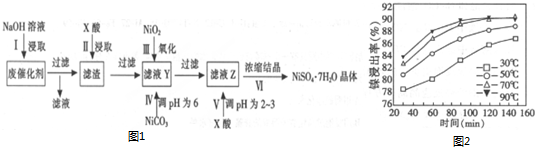
��������������������ʽ��ȫ����ʱ��pH���£�
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
��1����1������NaOH��Һ��Ŀ���dz�ȥAl��Al2O3����֬�����ʣ�
��2���������ʱ�������X�������ᣨ�ѧʽ������������������ͬ���ڲ�ͬ�¶��¶Է����������С������������������ʱ��仯��ͼ2����������������¶���ʱ��ֱ���C����ѡ����ĸ����
A.30�桢30min B.90�桢150min C.70�桢120min
��3����V����pHΪ2��3��Ŀ���ǣ����ϻ�ѧ�����ƽ���ƶ�ԭ�����ͣ���Һ�д���ˮ��ƽ��Ni2++2H2O?Ni��OH��2+2H+������������Ũ��ƽ��������б���Ũ��������ˮ�����ɳ�����
��4����ҵ��������NiOOHΪ�缫��NaOH��ҺΪ���Һ�Ƴɵ�أ��ŵ�ʱ��NiOOHת��ΪNi��OH��2���õ��������Ӧʽ��NiO��OH��+H2O+e-�TNi��OH��2+OH-��
��5����֪��2Mg��s��+O2��g��=2Mg��s����H1=-2075kJ/mol
Mg2Ni��s��+2MgH2��s��=2Mg��s��+Mg2NiH4��s����H2=+84.6kJ/mol
MgH2��s��+$\frac{1}{2}$O2��g��=MgO��s��+H2��g����H3=-963kJ/mol
����������ϣ�Mg2NiH4���ͷ�������Mg2Ni���Ȼ�ѧ����ʽ��Mg2NiH4��s��=Mg2Ni��s��+2H2��g����H=+64.4KJ/mol��
��6���ں����ϴ����У�Ni��Al��Fe�����������ֱ���29.5%��31%��5.6%��a kg�����ϴ�������������ת�����ڢ���cmol/L��X��bL����������ҺY����Fe3+����ҺZ�в���������Բ��ƣ���ڢ���Ӧ����NiCO30.119��cb-5.5a��kg��
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
��2������ЩԪ���У�����õĽ���Ԫ����K������õķǽ���Ԫ����F������õ�Ԫ����Ar��
��3������ЩԪ���У�ԭ�Ӱ뾶��С����F��ԭ�Ӱ뾶������K��
��4������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����KOH�������Ե�����������Al��OH��3��
| A�� | 2��2-����-1-������2-��-1-���� | |
| B�� | ���ȼױ������ȼױ� | |
| C�� | 2-����������� | |
| D�� | ����ϩ��ͼ������ |
| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
��2���ڢۢ�����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����Na��Mg��F ����Ԫ�ط��ű�ʾ��
��3���õ���ʽ��ʾ������γɻ�����Ĺ���
 ��
����4���ڢ١���Ԫ���У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4��������ǿ����NaOH�����ѧʽ��
��5���ڢ����ĵ����У���ѧ���ʽϻ��õ���Cl2���ѧʽ��������ʲô��ѧ��Ӧ˵����д����Ӧ�Ļ�ѧ����ʽ����Cl2+2Br-=2Cl-+Br2��
| A�� | H2��g��+Cl2��g���T2HCl��g����H=-184.6 kJ/mol | |
| B�� | CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802.3 kJ/mol | |
| C�� | C��s��+O2��g���TCO2��g����H=-393.5 kJ/mol | |
| D�� | 2H2��g��+O2��g���T2H2O��1����H=-571.6 kJ/mol |
 ���������л�ʵ�����г��õ���Ʒ������������ԭ��Ӧ֪ʶ�͵�ѧ֪ʶ���Լ��������һ��ԭ��أ�����д���пհף�
���������л�ʵ�����г��õ���Ʒ������������ԭ��Ӧ֪ʶ�͵�ѧ֪ʶ���Լ��������һ��ԭ��أ�����д���пհף�