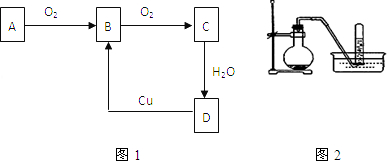
��Ŀ����
9����A��B��C��D��E��F��G����Ԫ�أ������������������ƶϣ���A��B��C��ͬһ���ڵĽ���Ԫ�أ���֪ԭ�Ӻ�����3�����Ӳ㣬A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶A��B��C��
��D��E�Ƿǽ���Ԫ�أ����Ǹ��⻯�Ͽ�������̬�⻯��HD��HE��������ʱ��D�ĵ�����Һ�壬E�ĵ����ǹ��壻
��F�ĵ����ڳ����������壬���ʺ��ȶ����dz�������������壻
��G�dz�����ԭ�Ӱ뾶��С��Ԫ�أ�
��1��A���������ƣ�Bλ�����ڱ��е������ڢ�A�壬C��ԭ�ӽṹʾ��ͼ��
 ��
����2��E�ĵ�����ɫ���Ϻ�ɫ��
��3��AԪ����DԪ���γɻ�����ĵ���ʽ��
 ��
����4��G�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��2F2+2H2O�T4HF+O2��
��5��F��Ԫ�ط�����He��
��6������������Ԫ���У�����������Ӧ��ˮ���������ǿ�Ļ�ѧʽ��NaOH��������ǿ�Ļ�ѧʽ��HBrO4����̬�⻯�����ȶ��Ļ�ѧʽ��HF��
��7����C���������Ӧ��ˮ����Ͷ�뵽A���������Ӧ��ˮ�����з�Ӧ�����ӷ���ʽ�ǣ�Al��OH��3+OH-�TAlO2-+2H2O��
���� ��A��B��C��ͬһ���ڵĽ���Ԫ�أ���֪ԭ�Ӻ�����3�����Ӳ㣬�����ڵ������ڣ�A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶A��B��C������֪AΪNa��BΪMg��CΪAl��
��D��E�Ƿǽ���Ԫ�أ����Ǹ��⻯�Ͽ�������̬�⻯��HD��HE��D��E����-1�ۣ����ߴ��ڢ�A�壬������ʱ��D�ĵ�����Һ�壬E�ĵ����ǹ��壬��DΪBr��EΪI��
��F�ĵ����ڳ����������壬���ʺ��ȶ����dz�������������壬��FΪHe��
��G�dz�����ԭ�Ӱ뾶��С��Ԫ�أ���GΪFԪ�أ�
��� �⣺��A��B��C��ͬһ���ڵĽ���Ԫ�أ���֪ԭ�Ӻ�����3�����Ӳ㣬�����ڵ������ڣ�A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶A��B��C������֪AΪNa��BΪMg��CΪAl��
��D��E�Ƿǽ���Ԫ�أ����Ǹ��⻯�Ͽ�������̬�⻯��HD��HE��D��E����-1�ۣ����ߴ��ڢ�A�壬������ʱ��D�ĵ�����Һ�壬E�ĵ����ǹ��壬��DΪBr��EΪI��
��F�ĵ����ڳ����������壬���ʺ��ȶ����dz�������������壬��FΪHe��
��G�dz�����ԭ�Ӱ뾶��С��Ԫ�أ���GΪFԪ�أ�
��1��������������֪��A���������ƣ�BΪMg��λ�����ڱ��е������ڢ�A�壬CΪAl��ԭ�ӽṹʾ��ͼ�� ��
��
�ʴ�Ϊ���ƣ�������A�� ��
��
��2��EΪI���䵥����ɫ���Ϻ�ɫ���ʴ�Ϊ���Ϻ�ɫ��
��3��AԪ����DԪ���γɻ�����ΪNaBr������ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��G�ĵ���Ϊ��������ˮ��Ӧ�Ļ�ѧ����ʽ�ǣ�2F2+2H2O�T4HF+O2���ʴ�Ϊ��2F2+2H2O�T4HF+O2��
��5��F��Ԫ�ط�����He���ʴ�Ϊ��He��
��6������������Ԫ���У�Na�Ľ�������ǿ������������Ӧ��ˮ���������ǿ�Ļ�ѧʽ��NaOH��F�ķǽ�������ǿ����û����ۺ����ᣬ����Ԫ����Br�ķǽ�������ǿ����������ǿ�Ļ�ѧʽ�� HBrO4����̬�⻯�����ȶ��Ļ�ѧʽ��HF���ʴ�Ϊ��NaOH��HBrO4��HF��
��7��C���������Ӧ��ˮ����Ϊ����������A���������Ӧ��ˮ����Ϊ�������ƣ����߷�Ӧ�����ӷ���ʽ�ǣ�Al��OH��3+OH-�TAlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-�TAlO2-+2H2O��
���� �����ۺ��Խ�ǿ�������֪ʶ��Ϲ㣬���Ƚϻ�������������Ԫ�ػ�����������Ԫ�������ɣ�ע��Ի���֪ʶ��ȫ�����գ�


| A�� | 25��ʱ��10-3mol/L�������pH=3��NH4Cl��Һ����pH=11�İ�ˮ�У�ˮ�ĵ���̶�Ϊ���ڣ��ۣ��� | |
| B�� | pH=1��NaHSO4��Һ��c��H+��=c��SO42-��+c��OH-�� | |
| C�� | pH=8.0��KHS��Һ�У�c��K+����c��HS-����c��OH-����c��S2-����c��H+�� | |
| D�� | ͼ��a����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c��OH-��=c��H+��+c��CH3COO-��+c��CH3COOH�� |
| A�� | 25��ʱ��pH=13�İ�ˮ��NaOH��Һ��1.0 L���е�OH-��Ŀ��Ϊ0.1NA | |
| B�� | ���³�ѹ�£���CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA | |
| C�� | 22 gD218O�����У��������������������ֱ�Ϊ10NA��12NA | |
| D�� | ��״���£�22.4 L CCl4�к��е�C-Cl����ĿΪ4NA |

| A�� | ���Դ����ΪA�� | |
| B�� | �����ĵ缫��Ӧ��2CH3OH+CO-2e-�T��CH3O��2CO+2H+ | |
| C�� | H+��������ͨ�����ӽ���Ĥ | |
| D�� | ���������������ռ������ڵ��� |
| A�� | ��Ư����Һ��ͨ��SO2��Ca2++ClO-+SO2+H2O�TCaSO3��+2HClO | |
| B�� | ��Fe��NO3��3��Һ�м��������HI��Һ��2Fe3++2I-�T2Fe2++I2 | |
| C�� | ��̼���Ⱶ��Һ�м������������������Һ��Ba2++2HCO3-+2OH-�TBaCO3��+CO32-+2H2O | |
| D�� | ��Na2S2O3��Һ�м���ϡ���S2O32-+4H+�TSO42-+3S��+2H2O |
 �����仯�����ڹ�ҵ���������������й㷺Ӧ�ã��ش��������⣺
�����仯�����ڹ�ҵ���������������й㷺Ӧ�ã��ش��������⣺��1�����������ȶ��������������������õ���ʽ��ʾ�������γɹ��̣�
 ��
����2��������N2H4����һ�ֻ�ԭ������֪��H2O��l��=H2O��g����H=+44kJ/mol���Խ���±����ݣ�д��N2H4 ��g��ȼ���ȵ��Ȼ�ѧ����ʽ��N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-631.7kJ/mol��
| ��ѧ�� | N-H | N-N | N=N | N��N | O=O | O-H |
| ���ܣ�kJ/mol�� | 390.8 | 193 | 418 | 946 | 497.3 | 462.8 |
��4�����Ĵ��������ڹ�ҵ�������ᣮ�÷�Ӧ����Ƴ����͵�أ���д�����Ի����£��õ�صĸ����缫��Ӧʽ��NH3-5e-+5OH-=NO+4H2O��
��5����ijŨ�ȵ�NO2�������һ���ݾ��������У�������Ӧ2NO2?N2O4�����ͼ�����£�
��0��3sʱv��NO2�������ԭ��������Ϊ������ϵ���÷�Ӧ������з��ȣ���ϵ�¶����ߣ�v��NO2������
��5sʱNO2ת����Ϊ75%��
| A�� | �٢ܢۢݢޢ� | B�� | �٢ݢۢܢޢ� | C�� | �٢ۢܢޢݢ� | D�� | �٢ۢݢޢܢ� |
 ��
�� ��
�� ��
��