��Ŀ����
������ҵ�ǹ��ҹ�ҵ�Ļ�������ش��йظ��������⣮
��1����ҵ���Ȼ�ԭ��������д����CO��ԭ��������Ҫ�ɷ�ΪFe2O3���Ļ�ѧ����ʽ��
�������к���������ʯ��SiO2�����������в���Ӱ�죬��Ҫ��ʯ��ʯ��ȥ��д����ȥ��ʯ�Ļ�ѧ����ʽ
��2�����������õ������к���̼���衢�ס�������ʣ�����ʱ��Ҫ��̼���������躬���������������Ļ�ѧ��Ӧ����ʽΪ
��3����֪��Fe��s��+
O2��g���TFeO��s����H=-272KJ/mol
2Al��s��+
O2��g���TAl2O3��s����H=-1675.7KJ/mol
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ�� ��
��1����ҵ���Ȼ�ԭ��������д����CO��ԭ��������Ҫ�ɷ�ΪFe2O3���Ļ�ѧ����ʽ��
�������к���������ʯ��SiO2�����������в���Ӱ�죬��Ҫ��ʯ��ʯ��ȥ��д����ȥ��ʯ�Ļ�ѧ����ʽ
��2�����������õ������к���̼���衢�ס�������ʣ�����ʱ��Ҫ��̼���������躬���������������Ļ�ѧ��Ӧ����ʽΪ
��3����֪��Fe��s��+
| 1 |
| 2 |
2Al��s��+
| 3 |
| 2 |
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��
���㣺��ѧ����ʽ����д,�ø�˹���ɽ����йط�Ӧ�ȵļ���
ר�⣺
��������1��һ����̼���л�ԭ�Կ��Ի�ԭ�������������Ͷ�����̼��
����������̼����ڸ����·�Ӧ���ɹ���ơ�������̼��
��2������������̼�ڸ����·�Ӧ���ɹ裻
��3�����������Ȼ�ѧ����ʽ���Σ����ø�˹���ɼ�����
����������̼����ڸ����·�Ӧ���ɹ���ơ�������̼��
��2������������̼�ڸ����·�Ӧ���ɹ裻
��3�����������Ȼ�ѧ����ʽ���Σ����ø�˹���ɼ�����
���
�⣺��1��һ����̼���л�ԭ�Կ��Ի�ԭ�������������Ͷ�����̼����ѧ����ʽΪ��3CO+Fe2O3
3CO2+2Fe��
����������̼����ڸ����·�Ӧ���ɹ���ơ�������̼����ѧ����ʽΪ��CaCO3+SiO2
CaSiO3+CO2��
�ʴ�Ϊ��3CO+Fe2O3
3CO2+2Fe��CaCO3+SiO2
CaSiO3+CO2��
��2������������̼�ڸ����·�Ӧ���ɹ裬��Ӧ�Ļ�ѧ����ʽΪ��SiO2+2C
Si+2CO����
�ʴ�Ϊ��SiO2+2C
Si+2CO����
��3����Fe��s��+
O2��g���TFeO��s����H=-272KJ/mol
��2Al��s��+
O2��g���TAl2O3��s����H=-1675.7KJ/mol
���ݸ�˹���ɢ�-�١�3�õ���3FeO��s��+2Al��s��=Al2O3��s��+3Fe��s����H=-859.7KJ/mol��
�ʴ�Ϊ��3FeO��s��+2Al��s��=Al2O3��s��+3Fe��s����H=-859.7KJ/mol��
| ||
����������̼����ڸ����·�Ӧ���ɹ���ơ�������̼����ѧ����ʽΪ��CaCO3+SiO2
| ||
�ʴ�Ϊ��3CO+Fe2O3
| ||
| ||
��2������������̼�ڸ����·�Ӧ���ɹ裬��Ӧ�Ļ�ѧ����ʽΪ��SiO2+2C
| ||
�ʴ�Ϊ��SiO2+2C
| ||
��3����Fe��s��+
| 1 |
| 2 |
��2Al��s��+
| 3 |
| 2 |
���ݸ�˹���ɢ�-�١�3�õ���3FeO��s��+2Al��s��=Al2O3��s��+3Fe��s����H=-859.7KJ/mol��
�ʴ�Ϊ��3FeO��s��+2Al��s��=Al2O3��s��+3Fe��s����H=-859.7KJ/mol��
���������⿼���˻�ѧ����ʽ���Ȼ�ѧ����ʽ����д����ȷ��Ӧ��ʵ���ǽ���ؼ���ע�����ø�˹������Ӧ�ȵķ�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����ʱ������˵���У���ȷ���ǣ�������
| A��0��l mol/L�������Һ�У�c��Ca2+����c��CH3COO-����c��OH-����c��H+�� |
| B�������pH����ͬ��NH4Cl��Һ�����ᣬ��ȫ�ܽ������ҵ�������п�ۣ�ǰ������ʱ���� |
| C����n mol H2��g����n mol I2��g����2n mol H2 ��g����2n mol I2��g���ֱ�����������º��ݵ������У�ƽ��ʱH2��ת����ǰ��С�ں��� |
| D��ij���ʵ���Һ����ˮ�������c��H+��=1��10-a mol?L-1����a��7ʱ�������Һ��pHһ��Ϊ14-a |
��������ĵ���ƽ�ⳣ�����������˵����ȷ���ǣ�������
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | Ki=1.77��10-4 | Ki=4.9��10-10 | Ki1=4.3��10-7 Ki2=5.6��10-11 |
| A��0.1 mol?L-lNaHCO3��Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c�� Na+����c��HCO3-��c��HJ-����c��OH-�� |
| B��������CO2ͨ�˵�NaCN��Һ�У�CN-+H2O+CO2�THCN+HCO3- |
| C����0.1 mol?L-l NaOH��Һ�к͵�Ũ�ȵ�HCN��Һ��HCOOH��Һʱ��ǰ������NaOH��Һ����� |
| D����ͬ�����0.1 mol?L-l HCOONa��Һ��0.1 mol?L-l NaCN��Һ������������С |
�������ʵ�ˮ��Һ�ܹ����磬�����ڷǵ���ʵ��ǣ�������
| A�����Ȼ�̼ | B���������� |
| C���Ȼ��� | D������ |
 ��ij�����ܱ������г���һ����C02��H����������Ӧ��CO2��g��+H2��g��?HCOOH��g�����ƽ����ϵ��C02�İٷֺ�����CQ%���뷴Ӧ�¶ȱ仯�Ĺ�ϵ��ͼ��ʾ������������ �У�a�����b����� ������Ӧ���ʢ��淴Ӧ���ʢ�HCOOH��g����Ũ�Ȣܶ�Ӧ�¶�ʱ��ƽ�ⳣ����������
��ij�����ܱ������г���һ����C02��H����������Ӧ��CO2��g��+H2��g��?HCOOH��g�����ƽ����ϵ��C02�İٷֺ�����CQ%���뷴Ӧ�¶ȱ仯�Ĺ�ϵ��ͼ��ʾ������������ �У�a�����b����� ������Ӧ���ʢ��淴Ӧ���ʢ�HCOOH��g����Ũ�Ȣܶ�Ӧ�¶�ʱ��ƽ�ⳣ����������| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
��ͼ��ʾ����H1=-393.5kJ?mol-1����H2=-395.4kJ?mol-1������˵�����ʾʽ��ȷ���ǣ�������


| A��C��s��ʯī���TC��s�����ʯ����H=+1.9 kJ?mol-1 |
| B��ʯī�ͽ��ʯ��ת���������仯 |
| C�����ʯ���ȶ���ǿ��ʯī |
| D��1 molʯī��������1 mol���ʯ���������� |
 ��������0.1mol?L-1������ҺNaOH��NH3?H2O��HCl��CH3COOH
��������0.1mol?L-1������ҺNaOH��NH3?H2O��HCl��CH3COOH һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����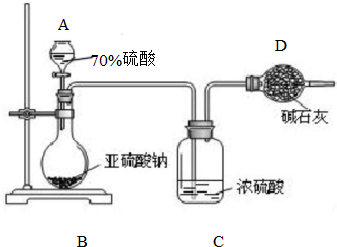 Ϊ�ⶨij����������Ʒ�Ĵ��ȣ���ͬѧ��ȡ10.0g���壬��������ʵ�飺
Ϊ�ⶨij����������Ʒ�Ĵ��ȣ���ͬѧ��ȡ10.0g���壬��������ʵ�飺