��Ŀ����
12������25��ʱ0.1mol/L�İ�ˮ����ش��������⣺������ˮ�м�����������粒��壬��ʱ��Һ��$\frac{C��O{H}^{-}��}{C��N{H}_{3}•{H}_{2}O��}$��С�����������С�����䡱����
������ˮ�м���ϡ���ᣬʹ��ǡ���кͣ�������Һ��pH�� 7���������������=�����������ӷ���ʽ��ʾ��ԭ��NH4++H2O?NH3•H2O+H+��
������ˮ�м���ϡ��������Һ��pH=7����ʱC��NH4+��=a mol/L����C��SO42-��Ϊ$\frac{a}{2}$mol/L��
���� ����ˮ�м�����������粒��壬笠�����Ũ���������ư�ˮ�ĵ��룬��Һ������������Ũ�ȼ�С����ˮ����Ũ������
������Ͱ�ˮ��Ӧ��������狀�ˮ���������ǿ��������ˮ���ʹ����Һ�����ԣ�
�۸�����Һ��������������������ȷ�����������Ũ�ȣ�
��� �⣺�����ڣ�NH4��2SO4=2NH4++SO42-����Һ��NH4+Ũ���������ư�ˮ���룬������Һ������������Ũ�ȼ�С����ˮ����Ũ���������Դ�ʱ��Һ��Һ��Һ��$\frac{C��O{H}^{-}��}{C��N{H}_{3}•{H}_{2}O��}$ ��С��
�ʴ�Ϊ����С��
������Ͱ�ˮ��Ӧ��������狀�ˮ���������ǿ��������ˮ���ʹ����Һ�����ԣ���������Һ��pH��7��ˮ�ⷽ��ʽΪ��NH4++H2O?NH3��H2O+H+��
�ʴ�Ϊ������NH4++H2O?NH3•H2O+H+��
����Һ�����ԣ�����Һ��c��H+��=c��OH-������Һ�ʵ����ԣ���Һ�д��ڵ���غ�c��NH4+��+c��H+��=2c��SO42- ��+c��OH-��������c��SO42- ��=$\frac{1}{2}$c��NH4+��=$\frac{a}{2}$mol/l��
�ʴ�Ϊ��$\frac{a}{2}$mol/l��
���� ���⿼����������ʵĵ��롢�����ˮ������Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬�ѶȲ�������Ũ�ȴ�С�ıȽ���ѧϰ���ѵ�Ҳ�ǿ��Ե��ȵ㣬���������غ�͵���غ������з������ɣ�

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д� ij��ѧС�����Na2SO3������ʵ��̽����
ij��ѧС�����Na2SO3������ʵ��̽������1���ڰ�ɫ��ΰ��a��b��c���������е���Na2SO3��Һ���ٷֱ�μ�ͼ��ʾ���Լ���
ʵ���������±���
| ��� | ʵ������ |
| a | ��ˮ��ɫ |
| b | ��������ɫ���� |
| c | �����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ |
��a��ʵ������֤��Na2SO3���л�ԭ���ԣ�
��b�з�����Ӧ�����ӷ���ʽ��SO32-+2S2-+6H+=3S��+3H2O��
��Ӧ�û�ѧƽ��ԭ������c�������û�ѧ���P�����ֱ�������Na2SO3��Һ�У�SO32-ˮ���Լ��ԣ�SO32-+H2O?HSO3-+OH-�����Ե����̪����Һ��죻�ڸ���Һ�м���BaCl2��Ba2++SO32-�TBaSO3������ɫ��������c��SO32-����С��SO32-ˮ��ƽ�����ƣ�c��OH-����С����ɫ��ȥ
��2������NaOH��Һ����SO2�Ĺ����У������õ�Na2SO3��NaHSO3�Ļ����Һ����ҺpH��n��SO${\;}_{3}^{2-}$����n��HSO${\;}_{3}^{-}$���仯��ϵ���±���
| n��SO${\;}_{3}^{2-}$ ����n��HSO${\;}_{3}^{-}$�� | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
A��c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-��
B��c��Na+����c��HSO3-����c��SO32-����c��OH-����c��H+��
C��c��Na+����c��SO32-����c��HSO3-����c��OH-����c��H+��
����n��SO32-����n��HSO3-��=3��2����0.8mol��NaOH��Һ�����˱�״���µ�SO211.2��L��
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | NaHCO3 | B�� | CH3COONa | C�� | NaCl | D�� | NH4Cl |
| A�� | ʯ�͵ķ����ѻ���ú��������Һ���������ǻ�ѧ�仯 | |
| B�� | ��ϩ�ͱ�������H2�����ӳɷ�Ӧ��˵�����߷���������̼̼����ͬ | |
| C�� | ���顢�����Ҵ������������������һ�������¶��ܷ���ȡ����Ӧ | |
| D�� | ���ۡ���֬�������ʶ��ܷ���ˮ�ⷴӦ����������Ȼ�л��߷��ӻ����� |
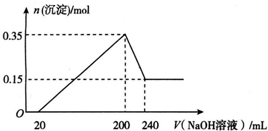 ��һ��������þ���������Ͷ��200 mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���������NaOH��Һ����ı仯��ϵ��ͼ��ʾ��������˵��������ǣ�������
��һ��������þ���������Ͷ��200 mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���������NaOH��Һ����ı仯��ϵ��ͼ��ʾ��������˵��������ǣ�������| A�� | þ������������Ϊ9 g | |
| B�� | ���20 mL NaOH��Һ�����к�����ϡ���� | |
| C�� | ����������Һ�����ʵ���Ũ��Ϊ5 mol•L-1 | |
| D�� | ���ɵ������ڱ�״���µ����Ϊ11.2 L |
| ��Ŀ | �۵�/��C | �ܶ�/ ��g•cm-3�� | Ӳ�ȣ���� ʯΪ10�� | ������ ����Ϊ100�� |
| ij�Ͻ� | 2 500 | 3.00 | 7.4 | 2.3 |
| �� | 1 535 | 7.86 | 4.5 | 17 |
| A�� | ���� | B�� | �Ŵ��� | C�� | ¯�� | D�� | �ɻ���� |

 �о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����壮
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����壮