��Ŀ����
16��ij��A��3��Ԫ����ɣ�������ˮ����A���ȣ����ɼȲ���ȼ��Ҳ��ʹʪ��ĺ�ɫ����ɫʯ����ֽ��ɫ������B��H2O����A����ˮ����������ʵ�飺��������������H2S�������ɵ���ɫ����C��ͬʱ�ų���ɫ����D��D�ڿ�����ת��Ϊ����ɫ����E���ڵ�ͨ������Cl2ʱ��������������������ҺŨ���ɵõ���ɫ������F����ش��������⣺��1��д��A�Ļ�ѧʽ��NH4NO2�� B�ĵ���ʽ��
 ��
����2��д������ʵ��١��������������ӷ�Ӧ����ʽ����2NO2-+2H++H2S=S��+2NO��+2H2O����NO2-+H2O+Cl2=NO3-+2Cl-+2H+��
��3��D��E�Ի�����Σ��������NaOH��Һ�����յ����ʵ�����D��E������壬д���仯ѧ��Ӧ����ʽNO2+NO+2NaOH=2NaNO2+H2O��
��4���ж���F�Ŀ��ܳɷ�NH4NO3��NH4Cl��NH4NO3��NH4Cl�Ļ������ʵ�鷽��ȷ��F�ijɷ�ȡmgF�����������������ữ��AgNO3��Һ������������������NH4Cl����ͨ������������������m֮��Ĺ�ϵ��ȷ���Ƿ���NH4NO3������������ֻ��NH4NO3��
���� ��������������H2S�������ɵ���ɫ����C�����ʣ�ͬʱ�ų���ɫ����һ��������һ�������ڿ�����ת��Ϊ����ɫ�������������˵��A���������Σ��ڵ�ͨ������Cl2ʱ��������������������ҺŨ���ɵõ���ɫ������F������Σ����A��3��Ԫ����ɣ�������ˮ����A���ȷֽ⣬����AΪ����泥����ȣ����ɼȲ���ȼ��Ҳ��ʹʪ��ĺ�ɫ����ɫʯ����ֽ��ɫ������B��H2O����B�ǵ������ɴ˷������
��� �⣺��������������H2S�������ɵ���ɫ����C�����ʣ�ͬʱ�ų���ɫ����һ��������һ�������ڿ�����ת��Ϊ����ɫ�������������˵��A���������Σ��ڵ�ͨ������Cl2ʱ��������������������ҺŨ���ɵõ���ɫ������F������Σ����A��3��Ԫ����ɣ�������ˮ����A���ȷֽ⣬����AΪ����泥����ȣ����ɼȲ���ȼ��Ҳ��ʹʪ��ĺ�ɫ����ɫʯ����ֽ��ɫ������B��H2O����B�ǵ�����
��1��A�Ļ�ѧʽ��NH4NO2��B�ĵ���ʽ�� ���ʴ�Ϊ��NH4NO2��
���ʴ�Ϊ��NH4NO2�� ��
��
��2����������������H2S�������ɵ���ɫ����C�����ʣ�ͬʱ�ų���ɫ����һ��������һ�������ڿ�����ת��Ϊ����ɫ�������������˵��A���������Σ���Ӧ�����ӷ���ʽΪ��2NO2-+2H++H2S=S��+2NO��+2H2O����NO2-+H2O+Cl2=NO3-+2 Cl-+2H+���ʴ�Ϊ��2NO2-+2H++H2S=S��+2NO��+2H2O��NO2-+H2O+Cl2=NO3-+2 Cl-+2H+����3��һ������������������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ��NO2+NO+2NaOH=2NaNO2+H2O���ʴ�Ϊ��NO2+NO+2NaOH=2NaNO2+H2O��
��4��Ũ���ɵõ���ɫ������F��������F����ΪNH4NO3��NH4Cl��NH4NO3��NH4Cl�Ļ�����ȡmg F�����������������ữ��AgNO3��Һ������������������NH4Cl����ͨ������������������m֮��Ĺ�ϵ��ȷ���Ƿ���NH4NO3������������ֻ��NH4NO3���ʴ�Ϊ��NH4NO3��NH4Cl��NH4NO3��NH4Cl�Ļ���ȡmg F�����������������ữ��AgNO3��Һ������������������NH4Cl����ͨ������������������m֮��Ĺ�ϵ��ȷ���Ƿ���NH4NO3������������ֻ��NH4NO3��
���� ���⿼����ε����ʣ��漰����ʽ����д����ѧ��Ӧ����ʽ����д�����ӷ���ʽ����д�Լ�ʵ�鷽������Ƶȣ��ۺ���ǿ����һ�����Ѷȣ�

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�| A�� | 2��3-����-3��3-���һ����� | B�� | 2-��-3-�һ����� | ||
| C�� | 2��3-����-1-��ϩ | D�� | 2��3-����-1-��Ȳ |

��֪��Cu2O+2H+=Cu2++Cu+H2O��
| A�� | ��������1���Ƴ��ù����ĩ��һ������NaNO3 | |
| B�� | ��������2���Ƴ��ù����ĩ��һ������K2SO4 | |
| C�� | ��������3���Ƴ��ù����ĩ��һ������K2SO3 | |
| D�� | ��������4���Ƴ��ù����ĩ��һ��û��Fe2O3 |
 25��ʱ��������ĵ���ƽ�ⳣ�����£�
25��ʱ��������ĵ���ƽ�ⳣ�����£�| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1 4.3��10-7 K2 5.6��10-11 | 3.0��10-8 |
��1��һ������£����¶�����ʱ��Ka�������������С�����䡱����
��2�������������ӽ�����������ɴ�С��˳����a��b��d��c������ţ���
a��CO32-b��ClO- c��CH3COO-d��HCO3-
��3�����з�Ӧ���ܷ�������cd������ţ�
a��CO32-+CH3COOH=CH3COO-+CO2��+H2O
b��ClO-+CH3COOH=CH3COO-+HClO
c��CO32-+2HClO=CO2��+H2O+2ClO-
d.2ClO-+CO2+H2O=CO32-+2HClO
��4��������ˮϡ��0.10mol•L-1�Ĵ��ᣬ���и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������b������ţ���
a.$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}��}$b.$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$ c.$\frac{c��{H}^{+}��}{{k}_{W}}$ d.$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$
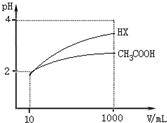
��5�������Ϊ10mL��pH��Ϊ2�Ĵ�����Һ��HX��Һ�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ��
��HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ�����ͬ������ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+�����ڴ�����Һ��ˮ���������c��H+����������ϡ�ͺ�HX��Һ�е�c��H+��С��CH3COOH��Һ�е�c��H+��������ˮ�ĵ������������������
��6��25��ʱ�������CH3COOH��CH3COONa�Ļ����Һ��pH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7 mol•L-1���ȷ��ֵ����
| A�� | ����NaOH��Һʱ���������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в����� | |
| B�� | ת�Ƶ�����ƿ�����У���������Һ���� | |
| C�� | ת�ƺ�δϴ��С�ձ��Ͳ����� | |
| D�� | ����ʱ���ӿ̶��� |
| A�� | 2 mol H2��g����1 mol I2��g�� | B�� | 3 mol HI��g�� | ||
| C�� | 2 mol H2��g����2 mol I2��g�� | D�� | 1 mol I2��g����2 mol HI��g�� |
 ��G��������ҵ���ܼ����ش��������⣺
��G��������ҵ���ܼ����ش��������⣺
 ����֪A��һ��ͬ���칹��Ҳ��ת��ΪB�����ͬ���칹��Ľṹ��ʽΪ
����֪A��һ��ͬ���칹��Ҳ��ת��ΪB�����ͬ���칹��Ľṹ��ʽΪ ��
�� +Br2��
+Br2�� ��
�� ��
�� ��
��