��Ŀ����
3���������������ɫ��Һ�壬�еȶ��ԣ����������ܷⱣ�棬�ش��������⣺������������Ϣ�жϣ�����˵������ȷ����AC������ĸ����
A�������������ȼ�գ�ȼ�ղ�����
B��������������Կ��������������ȡ������
C������������Ľṹ��ʽΪCH3COOCH2F
D������������ķе�ͣ��ӷ�
������������ĺϳɷ����кö��֣�����������һ��±�ؽ��������ϳɷ����������·��ͼ������Aͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��ʳ���е���Ҫ�ɷ֣�
�Իش��������⣺

��1��A��B��D���������У�����ԭ�Ӷ�λ��ͬһƽ���ϵ���CH2=CH2����ṹ��ʽ����B�����й����ŵ�����Ϊ�Ȼ���D��ͬϵ���У���Է���������С�����ʵĽṹ��ʽΪCH3OH��
��2���������ڻ�ѧ�仯���ٵķ�Ӧ�����ǷֽⷴӦ�����̢ڡ���������ȡ����Ӧ���Тۢߢ࣮
��3������E�п��ܻ��е�����C�ķ�����ȡ����E����̼��������Һ�������������ɣ�˵�����У�������
��4�������ʵ�����A��B��C��D��������������ȼ�գ��������������ɶൽ�ٵ�˳��ΪA��B��C=D������ĸ����
��5��д�����з�Ӧ�Ļ�ѧ����ʽ������ע����������
��CO+2H2��CH3OH��
��ClCH2COOH+CH3OH��ClCH2COOCH3+H2O��
���� ��I��A�����з�Ԫ�أ�ȼ�ջ������ж���HF��
B�����Կ���CH3COOCH3����Hԭ�ӱ�Fԭ��ȡ���õ�FCH2COOCH3��
C������������Ľṹ��ʽΪFCH2COOCH3��
D���������������ɫ��Һ�壬��Ҫ���������ܷⱣ�棬˵���е�ͣ�
��II��Aͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��ʳ���е���Ҫ�ɷ֣���BΪCH3COOH��B��PCl3��Ӧ����C��C��D��Ӧ����E����Eͨ��±�ؽ��������ϳɷ������������CΪClCH2COOH��DΪCH3OH��EΪClCH2COOCH3��
��� �⣺��I��A�����з�Ԫ�أ�ȼ�ջ������ж���HF����A����
B��������������Կ���CH3COOCH3����Hԭ�ӱ�Fԭ��ȡ���õ�FCH2COOCH3����B��ȷ��
C������������Ľṹ��ʽΪFCH2COOCH3����C����
D���������������ɫ��Һ�壬��Ҫ���������ܷⱣ�棬˵���е�ͣ��ӷ�����D��ȷ��
��ѡ��AC��
��II��Aͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��ʳ���е���Ҫ�ɷ֣���BΪCH3COOH��B��PCl3��Ӧ����C��C��D��Ӧ����E����Eͨ��±�ؽ��������ϳɷ������������CΪClCH2COOH��DΪCH3OH��EΪClCH2COOCH3��
��1��A��B��D���������У�����ԭ�Ӷ�λ��ͬһƽ���ϵ���CH2=CH2��BΪCH3COOH��B�����й����ŵ�����Ϊ�Ȼ���D��ͬϵ���У���Է���������С�����ʵĽṹ��ʽΪ��CH3OH��
�ʴ�Ϊ��CH2=CH2���Ȼ���CH3OH��
��2���������ڻ�ѧ�仯���ٵķ�Ӧ�����ǷֽⷴӦ�����̢ڡ���������ȡ����Ӧ���Тۢߢ࣬
�ʴ�Ϊ����ѧ���ֽ⣻�ۢߢࣻ
��3��C�к����Ȼ�������E�п��ܻ��е�����C�ķ����ǣ�ȡ����E����̼��������Һ�������������ɣ�˵�����У�������
�ʴ�Ϊ��ȡ����E����̼��������Һ�������������ɣ�˵�����У�������
��4��1molCH2=CH2����������2+$\frac{4}{4}$��mol=3mol��
1molCH3COOH����������2+$\frac{4}{4}$-$\frac{2}{2}$��mol=2mol��
ClCH2COOHȼ�����ɶ�����̼��ˮ��HCl����дΪCH2��CO2��HCl��1molClCH2COOH��������Ϊ��1+$\frac{2}{4}$��mol=1.5mol��
1mol CH3OHȼ����������Ϊ��1+$\frac{4}{4}$-$\frac{1}{2}$��mol=1.5mol��
�����������ɶൽ�ٵ�˳��Ϊ��A��B��C=D��
�ʴ�Ϊ��A��B��C=D��
��5����Ӧ�Ļ�ѧ����ʽΪ��CO+2H2��CH3OH��
��Ӧ�ߵĻ�ѧ����ʽΪ��ClCH2COOH+CH3OH��ClCH2COOCH3+H2O��
�ʴ�Ϊ��CO+2H2��CH3OH��ClCH2COOH+CH3OH��ClCH2COOCH3+H2O��
���� ���⿼���л���ĺϳɡ��л���Ľṹ�����ʡ�ú��ʯ�͵��ۺ����õȣ����ؿ���ѧ������������������Ϣ��ȡ�������Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�| A�� | �٢ڢܢ� | B�� | �٢ۢݢ� | C�� | �٢ۢܢ� | D�� | �٢ڢۢ� |
| A�� | 1.0LpH=12��CH3COONa��Һ��OH-һ��Ϊ0.01NA | |
| B�� | �����£�20g2H2O���еĵ�����Ϊ10NA | |
| C�� | mgNa2O2��ˮ��Ӧ����1molO2ʱת�Ƶ�����Ϊ2NA | |
| D�� | ��״���£�22.4LN2��CO�Ļ�����к��õ��Ӷ���ĿΪ2NA |
 �����ж�����Ԫ�صķ�������ȷ�ģ�������
�����ж�����Ԫ�صķ�������ȷ�ģ�������| A�� | ����������Ϊ3 | |
| B�� | ����������Ӧˮ����ķ���ʽ������H3RO4 | |
| C�� | ���Ϊ+3�� | |
| D�� | ��Ԫ�ؿ�����24��Ԫ�ظ���Cr����25��Ԫ���̣�Mn�� |
| A�� | �����ǿ��١�������ȷ�ⶨ��Է��������ķ��� | |
| B�� | ��������Dz������ڲⶨ������Ĺ����� | |
| C�� | �ú˴Ź���������1-������2-����� | |
| D�� | �������������ͬ���칹�� |
| A�� | �й��Ŵ����մ��������Ϊԭ�ϣ��������ս���ɵ� | |
| B�� | �Ŵ�Ⱦ������ij�֡������������˿�á����������Ҫ�ɷ��ǻ�� | |
| C�� | ������һ�գ���ˮ�����գ���ȡ֭���������϶������ص���ȡ���ڻ�ѧ�仯 | |
| D�� | ��ͥʹ�á�84������Һʱ��Ϊ��ǿ����Ч�������Լ������飨����ǿ�ᣩ |
| A�� | ȡ������ϡ����ĵ���ˮ��Һ���Թ��У����������NaOH��Һ���ٵμ�������ˮ�Լ�������Ƿ���ȫˮ�� | |
| B�� | ��AlCl3��Һ����εμ�NaOH��Һ����������֤��Al��OH��3�������������� | |
| C�� | ����к͵ζ�ʱ����ǿ��ζ�ǿ��ͨ���μ�2��3�μ�����ָʾ���������ȼ�������ʵ����Ӱ�� | |
| D�� | ��ɰֽ��ĥ������Ƭ�ϵμ��κ���̪�ı���ʳ��ˮ������1��2 min����۲쵽��Һ�εı�Եλ���ȳ��ֺ�ɫ |

 ��
��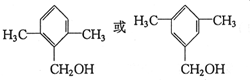 ��������һ�֣���
��������һ�֣���
 ��
�� ��д��A��B��Ԫ�ذ�1��1ԭ�Ӹ������γɻ�����ĵ���ʽ
��д��A��B��Ԫ�ذ�1��1ԭ�Ӹ������γɻ�����ĵ���ʽ ��
��