��Ŀ����
3��ij��ѧС��Ϊ��֤��SO2��Cl2��Ư���ԣ��������ͼ��ʾ��ʵ��װ�ã�
��1�������Ʊ�Cl2���ݵ�ԭ���ǣ�MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+2H2O+Cl2����Ӧѡ����ͼA��Eװ���е�E������ţ���Cl2��
��2����Ӧ��ʼ����B��D�����Թ��е�Ʒ����Һ����ɫ��ֹͣͨ����B��D�����Թ��е�Һ����ȣ�B�Թ��е���������Һ����ɫ��Ϊ��ɫ��
��3��װ��C�����������ն����SO2��Cl2��
��4����װ��B��D�е�Ʒ����Һ��������ɫʯ����Һ����B�Թ��е���������Һ��죮
��5��SO2��Cl2�������1��1��Ϻ�ͨ��ˮ�У��Ƿ���Ư���Է���ǡ���
������ԭ���ǣ��û�ѧ����ʽ��ʾ����SO2+Cl2+2H2O=H2SO4+2HCl��
���� ��1��ʵ�������ö���������Ũ�����ڼ����������Ʊ���������Ӧ�����Ȼ��̡�������ˮ����E�Ʊ���������A�Ʊ���������
��2�����������Ư���������ö����������ɫ���ʻ������ɲ��ȶ���ɫ���ʣ�������Һʱ�����±�ɺ�ɫ���������Ư���������ô������ǿ�����ԣ�������ɫ�����Һ���ܻظ���ɫ��
��3�����������������ж���ֱ���ŷŻ���Ⱦ������
��4������������ˮ���ϵõ������ᣬ�������ͨ�ԣ�����������Ư�����ָʾ����
��5��SO2��Cl2�������1��1��Ϻ�ͨ��ˮ�У�ǡ�÷�����SO2+Cl2+2H2O=H2SO4+2HCl��
��� �⣺��1��ʵ�������ö���������Ũ�����ڼ����������Ʊ���������Ӧ�����Ȼ��̡�������ˮ����Ӧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+2H2O+Cl2������E�Ʊ���������A�Ʊ���������
�ʴ�Ϊ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+2H2O+Cl2����E��
��2��Bװ���ж����������ɫ���ʻ������ɲ��ȶ���ɫ���ʣ�ʹƷ����Һ��ɫ��ȥ���ټ���B����ɫ����Һʱ���ֻظ���ɫ��
�ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ��
��3�����������������ж���ֱ���ŷŻ���Ⱦ������װ��C�������ǣ����ն����SO2��Cl2��
�ʴ�Ϊ�����ն����SO2��Cl2��
��4������������ˮ���ϵõ������ᣬ�������ͨ�ԣ�����������Ư�����ָʾ������Һ��ɫ��죬
�ʴ�Ϊ����Һ��죻
��5��SO2��Cl2�������1��1��Ϻ�ͨ��ˮ�У�ǡ�÷�����SO2+Cl2+2H2O=H2SO4+2HCl��û������ʣ�࣬û��Ư���ԣ�
�ʴ�Ϊ����SO2+Cl2+2H2O=H2SO4+2HCl��
���� ���⿼��������ʵ�����Ʊ���������Ͷ������������ʵ�飬�ѶȲ���ע��ѡ���Ʊ�������װ�ò�ͬ�����Ʊ�ԭ����ͬ���ؼ������Ƿ���ȣ�B����Һ��������Ҳ��ͬ��


| A�� | ͼ1��װ�ÿ����ڱȽ�MnO2��Cl2��S�������� | |
| B�� | ͼ2��֪��30��ʱCa��OH��2��Һ��Ũ��һ������60��ʱ���¶� | |
| C�� | ͼ3��װ�ÿ�������֪Ũ�ȵ�H2C2O4��Һ�ⶨδ֪Ũ�ȵ�NaOH��Һ | |
| D�� | ͼ4��ʵ���������Ը��������Һ�������ݳ��֣�����Һ��ɫ��dz������ȥ |
| A�� | ƽ��������Ӧ�����ƶ� | B�� | a+b��c+d | ||
| C�� | C������������� | D�� | B��ת�������� |
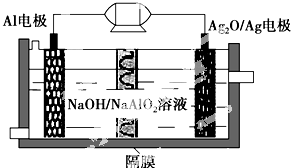 Al-Ag2O�����һ�ֿ�����ˮ�¶�����������Դ����ԭ����ͼ��ʾ���õ�ع���ʱ�ܷ�ӦʽΪ2Al+3Ag2O+2NaOH�T2NaAlO2+6Ag+H2O��������˵��������ǣ�������
Al-Ag2O�����һ�ֿ�����ˮ�¶�����������Դ����ԭ����ͼ��ʾ���õ�ع���ʱ�ܷ�ӦʽΪ2Al+3Ag2O+2NaOH�T2NaAlO2+6Ag+H2O��������˵��������ǣ�������| A�� | ����ʱ����������ԭ��Ӧ | |
| B�� | ���缫������1.08 g Agʱ����·��ת�Ƶĵ���Ϊ0.01 mol | |
| C�� | Al�缫�ķ�ӦʽΪAl-3e-+4OH-=AlO2-+2H2O | |
| D�� | ����ʱ���Һ�е�Na+����Ĥ����Al�缫�ŵ��� |
| A�� | ���ȵ���ʧȥ�ᾧˮ�Ĺ��̳Ʒ绯 | |
| B�� | �ڱ�����Һ�У������ܽ�����ʵ��ڽᾧ���� | |
| C�� | ԭ����ǰѵ���ת��Ϊ��ѧ�ܵ�װ�� | |
| D�� | �����Ƕ�����Դ |
��ע��������Լ�����д��ѧʽ����
| ���� | �����Լ� | ���ӷ���ʽ |
| NH4Cl��AlCl3����Һ | ||
| NaHCO3��Na2CO3����Һ | ||
| Fe �ۣ�Al�ۣ� | ||
| FeCl3��FeCl2����Һ |
 15����18�֣�
15����18�֣������������У�������ͨ����ϩ�ӳɷ�Ӧ�õ�����B������ţ���
A��CH3CH3B��CH3CHCl2
C��CH3CH2OHD��CH3CH2Br
����֪ 2CH3CHO+O2 $��_{��}^{����}$ 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
��Ӧ�ڵĻ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ
 ����Ӧ�����ǼӾ۷�Ӧ��
����Ӧ�����ǼӾ۷�Ӧ����ijͬѧ�����Ҵ���Ũ���Ṳ�ȵ���ϩ��̽�������з������¶ȹ��ߣ���ϩ�����п��ܺ��������Ķ������������������ʵ��Բ��������֤��

��1��A��B��C��Dװ�ÿ�ʢ�ŵ��Լ��ǣ�����������Ҫ���Լ��������ո��ڣ���
A�ܣ�B�٣�C�ܣ�D�ڣ�
��NaOH��Һ ��������Ȼ�̼��Һ ��Ũ���� ��Ʒ����Һ
��2��ȷ��������ϩ������Ϊ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽ��CH2=CH2+Br2��CH2Br-CH2Br
| A�� | ������ʴ�ĸ�����ӦΪ��Fe-3e-=Fe3+ | |
| B�� | Mg-Al��NaOH��Һ���ɵ�ԭ����и�������ΪMg | |
| C�� | ��Ĥ��ⱥ��NaCl��Һ���ò���֮һ�ǡ�84������Һ�е���Ч�ɷ�NaClO | |
| D�� | H2-O2ȼ�ϵ���е��ҺΪH2SO4����������ӦʽΪ��O2+4e-=2O2- |
| A�� | ��ZR��ɵĻ�����Ϊ���ӻ����� | B�� | ����������R��X | ||
| C�� | X��Yֻ�ܹ����ڹ��ۻ������� | D�� | ԭ�Ӱ뾶Z��R��Y��X |
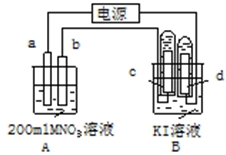 ��ͼװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݣ��Իش�
��ͼװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݣ��Իش�