��Ŀ����
2��������ʵ�����ӷ���ʽ�����Ӧ��ϵ����ȷ���ǣ�������| A�� | ������ϡ���ᣬ��Һ��Ϊdz��ɫFe+4H++NO3 --=Fe3++NO��+2H2O | |
| B�� | ��K2Cr2O7��Һ�м�����Ũ���ᣬ��Һ��Ϊ��ɫCr2O7 2-����ɫ��+H2O?2CrO4 2-����ɫ��+2H+ | |
| C�� | ����۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ����4H++4I-+O2=2I2+2H2O | |
| D�� | ϡ�İ�ˮ��Һ�����������Ķ�����̼����NH3•H2O+CO2=NH4++HCO3- |
���� A����ԭ�Ӹ������غ㣬��Һ��dz��ɫ˵�����ɶ��������ӣ�
B������ƽ���ƶ����۽��
C�������Ӿ���ǿ�Ļ�ԭ�ԣ��ܹ����������ɵ��ʵ⣻
D��������̼������Ӧ����̼��泥�
��� �⣺A��������ϡ���ᣬ��Һ��Ϊdz��ɫ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O����A����
B������������Ũ��ƽ�������ƶ�������Һ�ɻ�ɫ��Ϊ��ɫ����B����
C������۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ���������ӷ���ʽ��4H++4I-+O2=2I2+2H2O����C��ȷ��
D��ϡ�İ�ˮ��Һ�����������Ķ�����̼���壬���ӷ���ʽ��2NH3•H2O+CO2=2NH4++CO32-+H2O����D����
��ѡ��C��
���� ���⿼�������ӷ���ʽ����д����ȷ��Ӧʵ���ǽ���ؼ���ע���������ǿ�����ԣ�ע�ⷴӦ��ѭ����ʵ����ѭԭ�Ӹ����غ���ɣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
10����ѧ��ѧ�еij������ʼס��ҡ�������������ͼ��ʾת����ϵ����Ӧ����ʡ�ԣ�������˵����ȷ���ǣ�������

| A�� | ����Ϊͭ����Ϊ�Ȼ�����������һ�����Ȼ��� | |
| B�� | ����Ϊˮ����Ϊ�������ƣ����һ�������� | |
| C�� | ����Ϊ������Ϊ��������һ���������� | |
| D�� | ����Ϊþ����Ϊ����������һ������ |
17��E��F������Ԫ�أ���������һ����˵��������E����F���ǣ�������
| A�� | ������������E��F | |
| B�� | �����ʵ�����E��F�ֱ�������ϡ���ᷴӦ���������������ʵ�����E��F | |
| C�� | Ea+��Fb+����ͬ�ĵ��Ӳ�ṹ��a��b�� | |
| D�� | 25��ʱ��Ksp[E��OH��a]��Ksp[F��OH��b] |
14��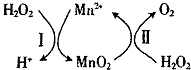 �̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�
�̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�
��1��п�̸ɵ�صķ�ӦΪ2MnO2+Zn+2NH4Cl=2MnO��OH��+Zn��NH3��2Cl2��MnO��OH������Ԫ�صĻ��ϼ�Ϊ+3��
��2����ϵ�ػ�ԭ��ķ�Һ������Mn2+��Fe2+��Zn2+�ȣ�����εμ�Na2S��Һ���������ɵij���ΪZnS���ѧʽ����[��֪Ksp��MnS��=1.4��10-15��Ksp��ZnS��=2.9��10 -25��Ksp��FeS��=6.0��10-18]
��3��Mn2+��H2O2�ֽ⣺2H2O2��l��=2H2O��l��+O2��g����H1���䷴Ӧ������ͼ��
����֪��Ӧ��ΪMnO2��s��+H2O2��1��+2H+ �� aq��=Mn2+ ��aq��+O2��g��+2H2O��1����H2��д����Ӧ I���Ȼ�ѧ����ʽ���ʱ��á�H1�͡�H2��ʾ����H2O2��1��+Mn2+��aq��=2H+��aq��+MnO2��s����H=��H1-��H2��
��ij�¶�ʱ����10mL0.4mol/L H2O2Һ�е���1��MnSO4�����ֽ⣺2H2O2=2H2O+O2����ò�ͬʱ������O2�������������Ϊ��״���µ�����������
0��2minʱ��Ӧ���ʱ�2��4minʱ�Ŀ죬��ԭ�������ŷ�Ӧ�Ľ��У�H2O2Ũ�Ȳ��ϼ�С����Ӧ���ʲ��ϼ�����0��6min��ƽ����Ӧ����v��H2O2��=3.3��10-2mol/��L•min����������Һ����ı仯����
��4���̻������Ǻϳɼ״��������ѵĴ�������֪��
�ٷ�ӦI������Ӧ�Ƿ��ȣ�����ȡ������ȡ�����Ӧ��
�ڷ�Ӧ���ƽ�ⳣ������ʽΪK=$\frac{c��C{H}_{3}OH����c��{H}_{2}O��}{c��C{O}_{2}����{c}^{3}��{H}_{2}��}$��
 �̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�
�̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ���1��п�̸ɵ�صķ�ӦΪ2MnO2+Zn+2NH4Cl=2MnO��OH��+Zn��NH3��2Cl2��MnO��OH������Ԫ�صĻ��ϼ�Ϊ+3��
��2����ϵ�ػ�ԭ��ķ�Һ������Mn2+��Fe2+��Zn2+�ȣ�����εμ�Na2S��Һ���������ɵij���ΪZnS���ѧʽ����[��֪Ksp��MnS��=1.4��10-15��Ksp��ZnS��=2.9��10 -25��Ksp��FeS��=6.0��10-18]
��3��Mn2+��H2O2�ֽ⣺2H2O2��l��=2H2O��l��+O2��g����H1���䷴Ӧ������ͼ��
����֪��Ӧ��ΪMnO2��s��+H2O2��1��+2H+ �� aq��=Mn2+ ��aq��+O2��g��+2H2O��1����H2��д����Ӧ I���Ȼ�ѧ����ʽ���ʱ��á�H1�͡�H2��ʾ����H2O2��1��+Mn2+��aq��=2H+��aq��+MnO2��s����H=��H1-��H2��
��ij�¶�ʱ����10mL0.4mol/L H2O2Һ�е���1��MnSO4�����ֽ⣺2H2O2=2H2O+O2����ò�ͬʱ������O2�������������Ϊ��״���µ�����������
| t/min | 0 | 2 | 4 | 6 |
| V��O2��mL | 0 | 9.9 | 17.2 | 22.4 |
��4���̻������Ǻϳɼ״��������ѵĴ�������֪��
| ��Ӧ | ƽ�ⳣ��KP | |
| 773K | 873K | |
| ��CO2��g��+4H2��g��?CH4��g��+2H2��g�� | 19.4 | 0.803 |
| ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� | 6.07��10-9 | 3.65��10-9 |
�ڷ�Ӧ���ƽ�ⳣ������ʽΪK=$\frac{c��C{H}_{3}OH����c��{H}_{2}O��}{c��C{O}_{2}����{c}^{3}��{H}_{2}��}$��
11�������£����и���������ָ����Һ���ܴ���������ǣ�������
| A�� | pH=3����Һ�У�K+��Ba2+��Cl-��Br- | |
| B�� | ����0.1mol•L-1Fe3+����Һ�У�K+��Mg2+��I-��SO42- | |
| C�� | ����0.1mol•L-1NO3-����Һ�У�Na+��H+��SO42-��Fe2+ | |
| D�� | ���д���AlO2-����Һ�У�NH4+��NO3-��Cl-��H+ |
12��������Ԫ��X��Y��Z��W��Q��ԭ����������������ֻ��һ�ֽ���Ԫ�أ�����X��W����ͬһ���壬ZԪ��ԭ�Ӱ뾶�ڶ����������ϡ��������⣩��W��Z֮����W��Q֮��ԭ������֮����ȣ�����Ԫ��ԭ������������֮��Ϊ21������˵����ȷ���ǣ�������
| A�� | Y�ļ���̬�⻯����һ�������¿ɱ�Q�������� | |
| B�� | Y�ļ����Ӱ뾶С��Z�ļ����Ӱ뾶 | |
| C�� | Q�ɷֱ���X��Y��Z��W�γɻ�ѧ��������ͬ�Ļ����� | |
| D�� | Z����������Ӧ��ˮ����ֱ���X��Y����������Ӧ��ˮ���ﷴӦ����1molˮʱ���ų���������ͬ |

 ��ѧ���ϵ��з���ʹ�ã�Ϊ����̫������Դ��Ѱ�÷�չ���¶����ṩ����֧�ţ����������ѧ֪ʶ�ش�
��ѧ���ϵ��з���ʹ�ã�Ϊ����̫������Դ��Ѱ�÷�չ���¶����ṩ����֧�ţ����������ѧ֪ʶ�ش�