��Ŀ����
 ��1�������£���20.0g 14%��NaCl��Һ��30.0g 24%��NaCl��Һ��ϣ��õ��ܶ�Ϊ1.17g/cm3�Ļ����Һ���û����Һ��NaCl�����ʵ���Ũ��Ϊ
��1�������£���20.0g 14%��NaCl��Һ��30.0g 24%��NaCl��Һ��ϣ��õ��ܶ�Ϊ1.17g/cm3�Ļ����Һ���û����Һ��NaCl�����ʵ���Ũ��Ϊ��2�����������������Դ���磨La��������Ni���ĺϽ����������ϣ�ͼΪ�úϽ�ľ���ṹ����С���ظ��ṹ��Ԫ����������һ����ԭ�ӣ�������ԭ�Ӷ������ϣ���ԭ�Ӷ��ڶ����ϣ��þ���Ļ�ѧʽΪ
��3��������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý���þ��ˮ���е�NO3-��ԭΪN2���Ӷ�������Ⱦ��
��д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ
��������Ӧ�У����ɱ�״����33.6L����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ
���㣺�����ļ���,���ʵ���Ũ�ȵ���ؼ���,������ԭ��Ӧ�ļ���
ר�⣺������ԭ��Ӧר��,��ѧ���뾧��ṹ
��������1�������Ϻ����������������c=
�����Ϻ�����ʵ���Ũ�ȣ�
��2���ṹ��̯�����㾧�����磨La��������Ni����ԭ����Ŀ���ݴ�˳��ѧʽ��
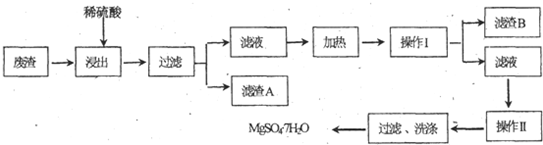
��3����þ���������Ӧ����������þ�������뵪�����ɵ���غ��֪���������������ӣ��ݴ���д��
�ڱ�״����33.6L���������ʵ���Ϊ1.5mol����Ԫ�ػ��ϼ���+5�۽���Ϊ0�ۣ��ݴ˼��㣻
����2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��֪����ҪMg0.3mol��2.5=0.75mol������þԪ���غ��֪���Ȼ�þ�����ʵ���Ϊ0.75mol�������Ȼ�þ��������������MgCl2�ĺ�ˮ����Ϊ�Ȼ�þ�����������Ȼ�þ������������
| 1000�Ѧ� |
| M |
��2���ṹ��̯�����㾧�����磨La��������Ni����ԭ����Ŀ���ݴ�˳��ѧʽ��
��3����þ���������Ӧ����������þ�������뵪�����ɵ���غ��֪���������������ӣ��ݴ���д��
�ڱ�״����33.6L���������ʵ���Ϊ1.5mol����Ԫ�ػ��ϼ���+5�۽���Ϊ0�ۣ��ݴ˼��㣻
����2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��֪����ҪMg0.3mol��2.5=0.75mol������þԪ���غ��֪���Ȼ�þ�����ʵ���Ϊ0.75mol�������Ȼ�þ��������������MgCl2�ĺ�ˮ����Ϊ�Ȼ�þ�����������Ȼ�þ������������
���
�⣺��1��20.0g 14%��NaCl��Һ��30.0g 24%��NaCl��Һ��ϣ���Ϻ��Ȼ��Ƶ���������Ϊ
=20%����Ϻ��Ȼ�����Һ���ܶ�Ϊ1.17g/ml�����Ի�Ϻ��Ȼ�����Һ�����ʵ���Ũ��Ϊ
=4.0mol/L��
�ʴ�Ϊ��4.0��
��2���ɾ����ṹͼ��֪���������磨La��ԭ����ĿΪ8��
=1������Ni����ԭ����ĿΪ1+8��
=5�����Ըþ���Ļ�ѧʽΪLaNi5��Ni5La��
�ʴ�Ϊ��LaNi5��Ni5La��
��3����þ���������Ӧ����������þ�������뵪�����ɵ���غ��֪���������������ӣ�þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ Ϊ2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��
�ʴ�Ϊ��2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��
�ڱ�״����33.6L���������ʵ���Ϊ
=1.5mol����Ԫ�ػ��ϼ���+5�۽���Ϊ0�ۣ�����ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��2����5-0��=15mol��
�ʴ�Ϊ��15��
����֪����þ���ԴӺ�ˮ����ȡ��MgCl2ͨ������Ƶõģ���ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO3-����2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��֪����ҪMg0.3mol��2.5=0.75mol������þԪ���غ��֪���Ȼ�þ�����ʵ���Ϊ0.75mol������������Ҫ��0.5%������������MgCl2�ĺ�ˮ����Ϊ
=14250g=14.25kg��
�ʴ�Ϊ��14.25��
| 20g��14%+30g��24% |
| 20g+30g |
| 1000��1.17�� 20% |
| 58.5 |
�ʴ�Ϊ��4.0��
��2���ɾ����ṹͼ��֪���������磨La��ԭ����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��LaNi5��Ni5La��
��3����þ���������Ӧ����������þ�������뵪�����ɵ���غ��֪���������������ӣ�þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ Ϊ2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��
�ʴ�Ϊ��2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��
�ڱ�״����33.6L���������ʵ���Ϊ
| 33.6L |
| 22.4L/mol |
�ʴ�Ϊ��15��
����֪����þ���ԴӺ�ˮ����ȡ��MgCl2ͨ������Ƶõģ���ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO3-����2NO3-+5Mg+6H2O=N2��+5Mg��OH��2+2OH-��֪����ҪMg0.3mol��2.5=0.75mol������þԪ���غ��֪���Ȼ�þ�����ʵ���Ϊ0.75mol������������Ҫ��0.5%������������MgCl2�ĺ�ˮ����Ϊ
| 0.75mol��95g/mol |
| 0.5% |
�ʴ�Ϊ��14.25��
���������⿼�����ʵ���Ũ�ȼ��㡢�������㡢��ѧ���P���ݷ���ʽ����ȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ��Ƕ�֪ʶ���ۺϿ��飬ע�⣨3���Тٳ����������������������þ��

��ϰ��ϵ�д�
�����Ŀ
������������������������������
��4HCl��Ũ��+MnO2
MnCl2+Cl2��+2H2O
��4HCl��g��+O2
2Cl2+2H2O��g��
��2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O
��������������ǿ������˳���ǣ�������
��4HCl��Ũ��+MnO2
| ||
��4HCl��g��+O2
| ||
| �� |
��2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O
��������������ǿ������˳���ǣ�������
| A��O2MnO2KMnO4 |
| B��KMnO4MnO2O2 |
| C��MnO2KMnO4O2 |
| D��O2KMnO4MnO2 |
25��ʱ���й�����ĵ��볣�����±�������˵����ȷ���ǣ�������
| ���� | CH3COOH | HCN | H2CO3 |
| Ka | 1.8��10-5 | 4.9��10-10 | K1��4.3��10-7 K2��5.6��10-11 |
| A��������ʵ���Ũ����ҺpH��ϵ��pH ��NaCN����pH ��Na2CO3����pH ��CH3COONa�� |
| B��������ʵ���Ũ�ȵ�HCN��NaCN�����Һ�У�c ��HCN��+c��H+��=c ��OH-��+c ��CN-�� |
| C��a mol/LHCN��b mol/LNaOH��Һ�������Ϻ����õ���Һ�У���c��Na+����c ��CN-������aһ��С��b |
| D����Na2CO3���뵽HCN��Һ��ʱ�������·�Ӧ��Na2CO3+HCN=NaCN+NaHCO3 |
�������ʵ�����F2��ClF��ϣ����ܱ������з�����Ӧ��F2��g��+ClF��g��?ClF3��g������H��0�����������У���ȷ���ǣ�������
| A�����º���ʱ����ClFת��40%ʱ�������ڵ�ѹǿΪ��ʼʱ��0.6 �� |
| B����c��F2����c��ClF����c��ClF3��=1��1��1����Ӧһ���ﵽƽ��״̬ |
| C���ﵽƽ��������������ݻ���������Ӧ���ʼ�С���淴Ӧ��������ƽ������ |
| D��ƽ����ٽ����¶ȣ����ֺ��ݣ��ﵽ�µ�ƽ�⣬���������ƽ��Ħ���������� |
�����£����и���������ָ����Һ�п��ܴ���������ǣ�������
| A��FeCl3��Һ�У�Al3+��K+��MnO4-��SO42- |
| B�����ȳʻ�ɫ����Һ�У�K+��Br-��S2-��ClO- |
| C���������۲�����������Һ�У�Na+��NH4+��NO3-��Cl- |
| D��Kw/c��OH-��=0.1mol/L����Һ�У�Na+��K+��AlO2-��CO32- |
mg�����к�����������Ϊa����ng�����к���ԭ����Ϊ��������
A��
| ||
B��
| ||
C��
| ||
D��
|
��������������þ������ͭ�Ļ����Һ�м���һ�������ۣ���ַ�Ӧ���ٹ��ˣ�������������ܴ��ڵ��ǣ�������
| A����ֽ����Ag��Cu����Һ����Ag+��Fe2+��Mg2+ |
| B����ֽ����Ag����Һ����Ag+��Cu2+��Fe2+��Mg2+ |
| C����ֽ����Ag��Cu����Һ����Fe2+��Mg2+ |
| D����ֽ����Ag��Cu��Fe����Һ����Fe2+��Mg2+ |