��Ŀ����
���û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��⣮ij�������Ƹ���ҵ������Cr�������������ù������£������Һ�н���������Ҫ��Cr3+�������Fe3+��Fe2+��Al3+��Ca2+��Mg2+��
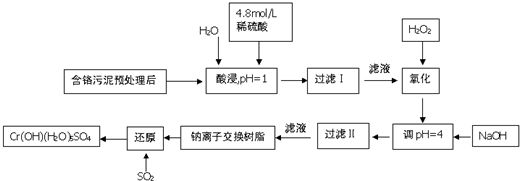
�����²��������ӵ����������γɳ���ʱ��Һ��pH���±���
��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�� ������һ����
��2����pH=4.0��Ϊ�˳�ȥ ����Fe3+��Al3+��Ca2+��Mg2+��
��3�������ӽ�����֬��ԭ��ΪMn++n NaR��MRn+nNa+�������������������� ����Fe3+��Al3+��Ca2+��Mg2+��
��4������ƽ����������ԭ��Ӧ����ʽ�� Na2Cr2O7+ SO2+ H2O= Cr��OH����H2O��5SO4+ Na2SO4��

�����²��������ӵ����������γɳ���ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Ca2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | 9.6 | 4.2 | 9.7 | - |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8.0 | 11.7 | 9.0����9.0�ܽ⣩ |
��2����pH=4.0��Ϊ�˳�ȥ
��3�������ӽ�����֬��ԭ��ΪMn++n NaR��MRn+nNa+��������������������
��4������ƽ����������ԭ��Ӧ����ʽ��
���㣺�����Ļ����뻷������Դ����,������ԭ��Ӧ����ʽ����ƽ,���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���
ר�⣺����ƽ������Һ��pHר��
��������1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ���ӳ���ȡʱ�䡢�ӿ��ܽ��ٶȵȴ�ʩ�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��������ܽ�����Ϊ����߽�ȡ�ʣ����������¶����������ܽ�ȣ�����Ӵ��������Ӧ���ʣ���ӿ�����ٶȵȣ�
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ����pH=4��Fe3+ת��Ϊ������ȥ��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӣ�
��4����������ͼ�е�ת����ϵ�Ͳ����϶�������Ļ�ԭ�ԣ�����������ԭ��Ӧԭ���жϣ�
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ����pH=4��Fe3+ת��Ϊ������ȥ��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӣ�
��4����������ͼ�е�ת����ϵ�Ͳ����϶�������Ļ�ԭ�ԣ�����������ԭ��Ӧԭ���жϣ�
���
�⣺��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�ǣ����ӽ�ȡʱ�䡢���Ͻ������������ν�ȡ�ȣ�
�ʴ�Ϊ�������¶ȣ����裬���˺����������м���H2SO4 ����ν�ȡ�����ʵ��ӳ���ȡʱ�䣻
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ�ʼ��ԣ���ҺPH=4��Fe3+��Al3+ת��Ϊ������ȥ��
�ʴ�Ϊ��Fe3+��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӣ���Ϊ�ڴ�֮ǰ��Fe3+����ȥ��Al3+ת��Ϊƫ���������ʽ���ʴ�Ϊ��Ca2+��Mg2+��
��4������������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2CrO4����Ϊ���ᣬNa2CrO4������ԭΪCrOH��H2O��5SO4��ˮ��Һ���������ᷴӦ���������ƣ�����ԭ���غ������д��ƽ��Na2CrO4+3SO2+11H2O=2CrOH��H2O��5SO4��+Na2SO4��
�ʴ�Ϊ��1��3��11��2��1��
�ʴ�Ϊ�������¶ȣ����裬���˺����������м���H2SO4 ����ν�ȡ�����ʵ��ӳ���ȡʱ�䣻
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ�ʼ��ԣ���ҺPH=4��Fe3+��Al3+ת��Ϊ������ȥ��
�ʴ�Ϊ��Fe3+��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӣ���Ϊ�ڴ�֮ǰ��Fe3+����ȥ��Al3+ת��Ϊƫ���������ʽ���ʴ�Ϊ��Ca2+��Mg2+��
��4������������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2CrO4����Ϊ���ᣬNa2CrO4������ԭΪCrOH��H2O��5SO4��ˮ��Һ���������ᷴӦ���������ƣ�����ԭ���غ������д��ƽ��Na2CrO4+3SO2+11H2O=2CrOH��H2O��5SO4��+Na2SO4��
�ʴ�Ϊ��1��3��11��2��1��
���������⿼�������ӷ���ʽ����ѧ����ʽ����д�����ʵķ����֪ʶ�㣬�ѶȽϴ�ע���������Һ��pHֵ����Һ�е����ӽ��з��룬���ӵ�ԭ���ǣ���ȥ�����Ҳ������µ����ʣ�

��ϰ��ϵ�д�
�����Ŀ
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ���ǣ�������
A����ͭ���缫��CuSO4����Һ��2Cu2++2H2O
| ||||
| B����NH4Fe��SO4��2��Һ�У��μ�����Ba��OH��2��Һ��2NH4++SO42-+Ba2++2OH-�T2NH3?H2O+BaSO4�� | ||||
| C����Na2CO3��Һ����ε���ϡ������Һ����Ӧ��ʼ�Σ�CO32-+H+�THCO3- | ||||
| D����Na2S2O3��Һ�м���ϡ���2S2O32-+4H+�TSO42-+3S��+2H2O |
�����������ʵ��ó��Ľ�����ȷ���ǣ�������
| A����ij��Һ�м���ϡ���ᣬ������ʹʯ��ˮ����ǵ����壬����Һһ������CO32- |
| B����ij��Һ�еμ���ˮ�μ�KSCN��Һ����Һ�ʺ�ɫ������Һ��һ������Fe2+ |
| C����ij��Һ�м����Ȼ�����Һ������������ϡ�����ɫ����������Һһ������SO42- |
| D����ij��Һ�м���NaOH���ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬����Һ��һ������NH4+ |




 ������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺