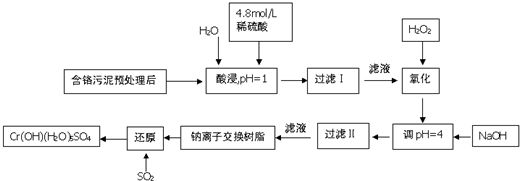
��Ŀ����
ij������ˮ��Һ�У�ֻ���ܺ������������е������֣�K+��Ca2+��NH4+��Cl-��CO32-��SO42-����ÿ��ȡ100.00mL����ʵ�飺
�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�������NaOH����ȣ��ռ�������0.896L����״���£�
�۵����ݼ�������BaCl2��Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��
��ش𣺣�1��c��CO32-��= ��
��2��K+�Ƿ���ڣ� �������ڣ�Ũ�ȷ�Χ�� ���������ڣ��ػش��2�ʣ� ��
�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�������NaOH����ȣ��ռ�������0.896L����״���£�
�۵����ݼ�������BaCl2��Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��
��ش𣺣�1��c��CO32-��=
��2��K+�Ƿ���ڣ�
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
�������ȸ���ʵ�������ж���Һ�д��ڵ����ӣ������ڵ����ӣ����ܴ��ڵ����ӣ�
��1�����жϢ�����ϴ��ǰ���������ٵ����ijɷ֣��ٸ��ݼ��ٵij�����������̼������ӵ�Ũ�ȣ�
��2����ȷ��ʵ�������ϴ������ijɷ֣�������������ӵ����ʵ��������ݰ������������笠����ӵ����ʵ������ٸ�����Һ�������������������ȣ��жϼ������Ƿ���ڣ������ڣ������������ӵĵ����ȣ���������ӵ����ʵ������ٸ������ʵ���Ũ�ȹ�ʽ��������ӵ����ʵ���Ũ�ȣ�
��1�����жϢ�����ϴ��ǰ���������ٵ����ijɷ֣��ٸ��ݼ��ٵij�����������̼������ӵ�Ũ�ȣ�
��2����ȷ��ʵ�������ϴ������ijɷ֣�������������ӵ����ʵ��������ݰ������������笠����ӵ����ʵ������ٸ�����Һ�������������������ȣ��жϼ������Ƿ���ڣ������ڣ������������ӵĵ����ȣ���������ӵ����ʵ������ٸ������ʵ���Ũ�ȹ�ʽ��������ӵ����ʵ���Ũ�ȣ�
���
�⣺����ʵ��������жϣ�����Һ�п��ܺ���Cl-��CO32-��SO42-��
����ʵ��������жϣ�����Һ�к���NH4+��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ�����Ca2+��
��1������ʵ���������ϴ�ӳ���ǰ���������٣����ٵ�����Ϊ̼�ᱵ��������
Ba2++CO32-=BaCO3��
1mol 197g
0.02mol ��6.27-2.33��g��
̼��������ʵ���Ũ��=
=0.2mol/L
�ʴ�Ϊ��0.2mol/L��
��2������ʵ���������ϴ�ӳ��������������Ϊ���ᱵ��������
Ba2++SO42-=BaSO4��
1mol 233g
0.01mol 2.33g
笠����ӵ����ʵ���Ϊ
NH4++OH-
NH3��+H2O
1mol 22.4L
0.04m0l 0.896L
������Һ�������������������ȵã������Ӵ��ڣ�һ�������Ӵ�һ����λ�ĸ���ɣ�һ����������ӡ�һ��̼������Ӷ�����������λ�ĸ���ɣ�һ��笠����ӡ�һ�������Ӹ���һ����λ������ɣ�
������Һ���������������������з���ʽ��
0.02mol��2+0.01mol��2+n��Cl-����1=0.04m0l��1+n��K+����1
n��K+��=0.02mol+n��Cl-�����������Ӳ���ȷ���Ƿ���ڣ���
�����ӵ����ʵ���Ũ�ȡ�
=0.2mol/L
�ʴ�Ϊ�����ڣ���0.2mol/L��
����ʵ��������жϣ�����Һ�к���NH4+��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ�����Ca2+��
��1������ʵ���������ϴ�ӳ���ǰ���������٣����ٵ�����Ϊ̼�ᱵ��������
Ba2++CO32-=BaCO3��
1mol 197g
0.02mol ��6.27-2.33��g��
̼��������ʵ���Ũ��=
| 0.02mol |
| 0.1L |
�ʴ�Ϊ��0.2mol/L��
��2������ʵ���������ϴ�ӳ��������������Ϊ���ᱵ��������
Ba2++SO42-=BaSO4��
1mol 233g
0.01mol 2.33g
笠����ӵ����ʵ���Ϊ
NH4++OH-
| ||
1mol 22.4L
0.04m0l 0.896L
������Һ�������������������ȵã������Ӵ��ڣ�һ�������Ӵ�һ����λ�ĸ���ɣ�һ����������ӡ�һ��̼������Ӷ�����������λ�ĸ���ɣ�һ��笠����ӡ�һ�������Ӹ���һ����λ������ɣ�
������Һ���������������������з���ʽ��
0.02mol��2+0.01mol��2+n��Cl-����1=0.04m0l��1+n��K+����1
n��K+��=0.02mol+n��Cl-�����������Ӳ���ȷ���Ƿ���ڣ���
�����ӵ����ʵ���Ũ�ȡ�
| 0.02mol |
| 0.1L |
�ʴ�Ϊ�����ڣ���0.2mol/L��
���������⿼�������ӵļ��鷽�������ӹ���֪ʶ�������������������������ǽ⣨2���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
���й����У����ۼ����ƻ����ǣ�������
| A��HCl��������ˮ�� |
| B�������� |
| C������������ˮ |
| D��������������̿���� |
һ����ɫ��ĩ���ܺ���Al��NO3��3��KOH��NaCl��һ�ֻ���֣�ij�о���ѧϰС��Ϊ��̽���ð�ɫ��ĩ����ɣ�������������ʵ�飺
��һ�������÷�ĩ��ˮ�ܽ⣬�õ�������Һ��
�ڶ�����ȡ�ó�����Һ��������μ����������������Ȳ�����ɫ������������ܽ⣻
��������ȡ�ڶ���������Һ����������AgNO3��Һ���а�ɫ�������֣�
�������ж���ȷ���ǣ�������
��һ�������÷�ĩ��ˮ�ܽ⣬�õ�������Һ��
�ڶ�����ȡ�ó�����Һ��������μ����������������Ȳ�����ɫ������������ܽ⣻
��������ȡ�ڶ���������Һ����������AgNO3��Һ���а�ɫ�������֣�
�������ж���ȷ���ǣ�������
| A���÷�ĩ��һ������Al��NO3��3��KOH��NaCl |
| B���÷�ĩ��һ������Al��NO3��3����KOH��������ȷ���Ƿ���NaCl |
| C���÷�ĩ��һ������NaCl��������ȷ���Ƿ���Al��NO3��3����KOH |
| D�����Ϲ��̲���ȷ������Һ�к����������� |
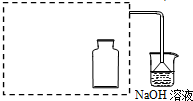
 ��1����ͼ��ʾ������һƿ������ˮ������ˮ���У����չ����䵽ʢ����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���ǣ�������صķ�Ӧ����ʽ�ͼ�Ҫ����˵����
��1����ͼ��ʾ������һƿ������ˮ������ˮ���У����չ����䵽ʢ����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���ǣ�������صķ�Ӧ����ʽ�ͼ�Ҫ����˵����