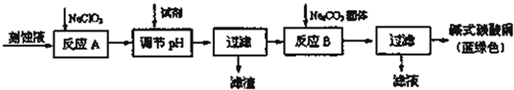
��Ŀ����
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϣ���ʳ�γ���������K+��Ca2+��Mg2+��SO42-���������ӣ�ʵ�����ᴿNaCl��������ͼ1��ʾ���ṩ���Լ��У�����Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ��75%�Ҵ������Ȼ�̼��
������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ���ѧʽ����
�ڲ������������ ��
��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ ������NaCl�Ƿ�ϴ���ķ����� ��
�����ᴿ��NaCl����480mL 4.00mol/L��NaCl��Һ����Ҫ��ȡNaCl������Ϊ g������������ҩ�ס���ƽ�����������ձ������ �����������ƣ���
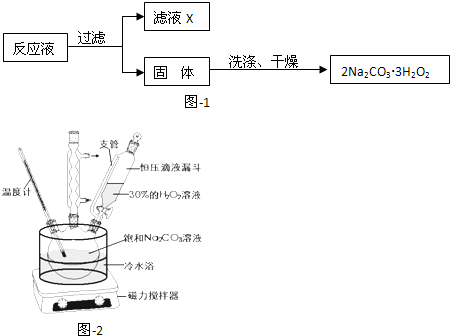
�ݵ�ⱥ��ʳ��ˮ��װ����ͼ2��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2 �����������=��������2L��ԭ���� ��

������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ
�ڲ������������
��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ
�����ᴿ��NaCl����480mL 4.00mol/L��NaCl��Һ����Ҫ��ȡNaCl������Ϊ
�ݵ�ⱥ��ʳ��ˮ��װ����ͼ2��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ����,ʵ�������
���������μ����ܽ⣬��ȥ��ʳ�γ���������K+��Ca2+��Mg2+��SO42-���������ӣ���BaCl2��Һ��ȥSO42-����NaOH��ȥMg2+���ñ���Na2CO3��Һ��ȥCa2+��Ȼ����ˣ��õ���������Һ�������ɷ�ΪBaSO4��Mg��OH��2��CaCO3������Һ�м���ϡ���ᣬ��ȥ�����ı���Na2CO3��Һ��������Һ��pH���õ�NaCl��Һ��Ȼ�����Ũ������ȴ�ᾧ�õ��Ȼ��ƾ��壬Ȼ��ϴ�ӡ�����õ���NaCl���ݴ˷������
���
�⣺���μ����ܽ⣬��ȥ��ʳ�γ���������K+��Ca2+��Mg2+��SO42-���������ӣ���BaCl2��Һ��ȥSO42-����NaOH��ȥMg2+���ñ���Na2CO3��Һ��ȥCa2+��Ȼ����ˣ��õ���������Һ�������ɷ�ΪBaSO4��Mg��OH��2��CaCO3������Һ�м���ϡ���ᣬ��ȥ�����ı���Na2CO3��Һ��������Һ��pH���õ�NaCl��Һ��Ȼ�����Ũ������ȴ�ᾧ�õ��Ȼ��ƾ��壬Ȼ��ϴ�ӡ�����õ���NaCl��
������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ��������Ϸ���֪��a���������Լ������μ�˳������ΪBaCl2��NaOH��Na2CO3���ʴ�Ϊ��BaCl2��NaOH��Na2CO3��
�ڴ���Һ����ȡNaCl�����ü���Ũ���ķ��������Բ�����������Ǽ���Ũ�����ʴ�Ϊ������Ũ����
��NaCl�������Ҵ����Ҵ��ӷ�������ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ75%�Ҵ���NaCl��KCl��������ˮ����KԪ����ɫ��Ӧ����ɫ��NaԪ����ɫ��Ӧ�ʻ�ɫ�����Լ���NaCl�Ƿ�ϴ���ķ������øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻������ɫ���棬��˵����ϴ����
�ʴ�Ϊ��75%�Ҵ����øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻������ɫ���棬��˵����ϴ����
��ʵ����û��480mL����ƿ����500mL����ƿ������Ҫ����500mL��Һ����ҪNaCl����=4.00mol/L��0.5L��58.5g/mol=117g��
����������ҩ�ס���ƽ�����������ձ�������Ҫ�������õĽ�ͷ�ιܡ�������Һ��500mL����ƿ��
�ʴ�Ϊ��117����ͷ�ιܡ�500mL����ƿ��
�ݵ�ⱥ��ʳ��ˮ����ʽΪ2NaCl+2H2O
2NaOH+Cl2��+H2�������ݷ���ʽ֪�����ռ���H2Ϊ2L����Ӧ���ռ�2L������������������ˮ������ͬ���������ռ���Cl2��2L��
�ʴ�Ϊ��������������ˮ�����ռ��������ƫС��
������ȥ��Һ���е�Ca2+��Mg2+��SO42-���ӣ��������Ϸ���֪��a���������Լ������μ�˳������ΪBaCl2��NaOH��Na2CO3���ʴ�Ϊ��BaCl2��NaOH��Na2CO3��
�ڴ���Һ����ȡNaCl�����ü���Ũ���ķ��������Բ�����������Ǽ���Ũ�����ʴ�Ϊ������Ũ����
��NaCl�������Ҵ����Ҵ��ӷ�������ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ75%�Ҵ���NaCl��KCl��������ˮ����KԪ����ɫ��Ӧ����ɫ��NaԪ����ɫ��Ӧ�ʻ�ɫ�����Լ���NaCl�Ƿ�ϴ���ķ������øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻������ɫ���棬��˵����ϴ����
�ʴ�Ϊ��75%�Ҵ����øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻������ɫ���棬��˵����ϴ����
��ʵ����û��480mL����ƿ����500mL����ƿ������Ҫ����500mL��Һ����ҪNaCl����=4.00mol/L��0.5L��58.5g/mol=117g��
����������ҩ�ס���ƽ�����������ձ�������Ҫ�������õĽ�ͷ�ιܡ�������Һ��500mL����ƿ��
�ʴ�Ϊ��117����ͷ�ιܡ�500mL����ƿ��
�ݵ�ⱥ��ʳ��ˮ����ʽΪ2NaCl+2H2O
| ||
�ʴ�Ϊ��������������ˮ�����ռ��������ƫС��
���������⿼������ķ�����ᴿ�����ؿ���ѧ���������⡢�����������ܴ������Ϸ����������̷����ķ�Ӧ��ʵ��������Ƽ�˳��ע������ƿѡȡ������Ϊ�״��㣮

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����������ҺŨ��ƫ�͵�ԭ���ǣ�������������ȷ����������
| A���ܽ��δ��ȴ�����¾���������ƿ |
| B��������ƿ��ˮʱҺ����ڿ̶���ҡ�� |
| C������ʱ����� |
| D��������ƿ��ˮ����ʱ�۾����ӿ̶��� |
CaCO3��ϡ���ᷴӦ�����ȷ�Ӧ������CO2�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ�����н��۲���ȷ���ǣ�������


| A����Ӧ��ʼ2������ƽ����Ӧ������� |
| B����Ӧ4���Ӻ�ƽ����Ӧ������С |
| C����Ӧ��ʼ4�������¶ȶԷ�Ӧ���ʵ�Ӱ���Ũ�ȴ� |
| D����Ӧ�ڵ�2min����4min������CO2��ƽ����Ӧ������� |