��Ŀ����
ij������;�㷺�������������Լ���ýȾ��������������ԭ�ϣ����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol��Ϊ��һ��ȷ��������ɣ�ij��ѧ��ȤС����������ʵ�飺
��ȡ48.20g����������ˮ�����100mL��Һ��������Һ���ػ�ɫ��
��ȡ������Һ50mL���Թ��У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壮
����ȡ������Һ50mL���Թ��У�����������BaCl2��Һ����������������İ�ɫ���� 23.30g��
��ش��������⣺
��1��ʵ����в�������ĵ���ʽ ��
��2�������ʵĻ�ѧʽΪ �������йظ����ʵ���;�������� ��
A����Ѫ�� B����ˮ�� C�����ӷ�ˮ�ļ���Լ� D������
��3����������Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽ ��
��4����SO2����ͨ������ʵ���Һ�п��Թ۲쵽��ʵ�������� ��д���÷�Ӧ�����ӷ���ʽ ��
��5��Ϊ�˽�һ����֤����������Ԫ�صĻ��ϼۣ�ijͬѧ���������ʵ�鷽����ȡ�����������Թ��У���ˮ����ܽ⣬�μ����軯����Һ������Һ��Ѫ��ɫ������֤����������һ������Fe3+��
�����۸�ʵ������Ƿ���� ������������ߡ����������������� ��
��ȡ48.20g����������ˮ�����100mL��Һ��������Һ���ػ�ɫ��
��ȡ������Һ50mL���Թ��У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壮
����ȡ������Һ50mL���Թ��У�����������BaCl2��Һ����������������İ�ɫ���� 23.30g��
��ش��������⣺
��1��ʵ����в�������ĵ���ʽ
��2�������ʵĻ�ѧʽΪ
A����Ѫ�� B����ˮ�� C�����ӷ�ˮ�ļ���Լ� D������
��3����������Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽ
��4����SO2����ͨ������ʵ���Һ�п��Թ۲쵽��ʵ��������
��5��Ϊ�˽�һ����֤����������Ԫ�صĻ��ϼۣ�ijͬѧ���������ʵ�鷽����ȡ�����������Թ��У���ˮ����ܽ⣬�μ����軯����Һ������Һ��Ѫ��ɫ������֤����������һ������Fe3+��
�����۸�ʵ������Ƿ����
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
������48.20g�����ʵ����ʵ�����
=0.1mol�����100mL��Һ�����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol������������ˮ������Һ���ػ�ɫ������Fe3+��ȡ������Һ50mL����0.05mol�����У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ��������ʵ�����
=0.05mol�����Ժ���笠�������0.05mol����1mol�����к���笠�����1mol�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壬��ΪFe2O3�����ʵ�����
=0.025mol��������������0.05mol������1mol�����к���������1mol��0.05mol�������м���������BaCl2��Һ����������������İ�ɫ���������ᱵ 23.30g����0.1mol��1mol���������Ժ�����������ӵ����ʵ�����2mol��������ʵ�Ħ���������õ������ʵķ���ʽΪ��NH4Fe��SO4��2?12H2O���������ʵ��������شɣ�
| 48.2g |
| 482g/mol |
| 0.85g |
| 17g/mol |
| 4.00g |
| 160g/mol |
���
�⣺48.20g�����ʵ����ʵ�����
=0.1mol�����100mL��Һ�����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol������������ˮ������Һ���ػ�ɫ������Fe3+��ȡ������Һ50mL����0.05mol�����У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ��������ʵ�����
=0.05mol�����Ժ���笠�������0.05mol����1mol�����к���笠�����1mol�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壬��ΪFe2O3�����ʵ�����
=0.025mol��������������0.05mol������1mol�����к���������1mol��0.05mol�������м���������BaCl2��Һ����������������İ�ɫ���������ᱵ 23.30g����0.1mol��1mol���������Ժ�����������ӵ����ʵ�����2mol��������ʵ�Ħ���������õ������ʵķ���ʽΪ��NH4Fe��SO4��2?12H2O��
��1�������У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ����������ĵ���ʽΪ��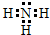 ���ʴ�Ϊ��
���ʴ�Ϊ��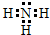 ��
��
��2�����ʵĻ�ѧʽΪNH4Fe��SO4��2?12H2O�������������ӷ�ˮ�ļ���Լ��������Ϸ��ϣ��ʴ�Ϊ��NH4Fe��SO4��2?12H2O��CD��
��3��NH4Fe��SO4��2?12H2O��Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽΪ��NH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3�����ʴ�Ϊ��NH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3����
��4�������Ӿ��������ԣ��ܽ�����������������Ӧ�ķ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Һ���ػ�ɫ��Ϊdz��ɫ��
�ʴ�Ϊ����Һ���ػ�ɫ��Ϊdz��ɫ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��5�������������Ӿ��л�ԭ�ԣ���ˮ����ܽ⣬�ܱ�ˮ�е���������Ϊ�����ӣ������������ӵļ��飬Ӧ������еķ�����ȥ������ͬʱ�ټ������ữ����ֹ���ʣ����Ը÷�����������
�ʴ�Ϊ����������������Һʱ���ܽ����õ�ˮ��Ҫ�ȼ�����г�ȥ�ܽ��������ͬʱ��Һ�ټ�ϡ�����ữ��
| 48.2g |
| 482g/mol |
| 0.85g |
| 17g/mol |
| 4.00g |
| 160g/mol |
��1�������У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ����������ĵ���ʽΪ��
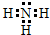 ���ʴ�Ϊ��
���ʴ�Ϊ��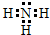 ��
����2�����ʵĻ�ѧʽΪNH4Fe��SO4��2?12H2O�������������ӷ�ˮ�ļ���Լ��������Ϸ��ϣ��ʴ�Ϊ��NH4Fe��SO4��2?12H2O��CD��
��3��NH4Fe��SO4��2?12H2O��Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽΪ��NH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3�����ʴ�Ϊ��NH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3����
��4�������Ӿ��������ԣ��ܽ�����������������Ӧ�ķ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Һ���ػ�ɫ��Ϊdz��ɫ��
�ʴ�Ϊ����Һ���ػ�ɫ��Ϊdz��ɫ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��5�������������Ӿ��л�ԭ�ԣ���ˮ����ܽ⣬�ܱ�ˮ�е���������Ϊ�����ӣ������������ӵļ��飬Ӧ������еķ�����ȥ������ͬʱ�ټ������ữ����ֹ���ʣ����Ը÷�����������
�ʴ�Ϊ����������������Һʱ���ܽ����õ�ˮ��Ҫ�ȼ�����г�ȥ�ܽ��������ͬʱ��Һ�ټ�ϡ�����ữ��
����������Ŀ��һ��ʵ�鷽����Ƶ�ʵ��̽���⣬����ѧ�������ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
ij�¶��£�V mL������NaNO3��Һa g��������b gˮ�����b g NaNO3���壨�ָ���ԭ�¶ȣ�����ʹ��Һ�ﵽ���ͣ����������ļ�������ȷ���ǣ�������
| A�����¶���NaNO3���ܽ��Ϊ200 g | ||
B��ԭ��������Һ��NaNO3����������Ϊ
| ||
C��ԭ��������Һ��NaNO3�����ʵ���Ũ��Ϊ
| ||
D��ԭ��������Һ���ܶ�Ϊ
|
�������ӷ���ʽ��ȷ���ǣ�������
| A��ʯ��ˮ�����̼��������Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O | ||||
B�����ˮ�еμӱ��͵�FeCl3��Һ�Ʊ�Fe��OH��3���壺Fe3++3H2O����ˮ��
| ||||
| C��������ͭ��ϡ���ᷴӦ��H++OH-�TH2O | ||||
| D��̼��������Һ�м������CO32-+2H+�TCO2��+H2O |
ƫ�����£�C2H8N2����һ�ָ���ȼ�ϣ�ȼ�ղ����ľ���������Ϊ�������ػ�����ƶ����������й�ƫ�����£�C2H8N2��������ȷ���ǣ�������
| A��ƫ�����µ�Ħ������Ϊ60g |
| B��1 molƫ�����µ�����Ϊ60g/mol |
| C��6.02��1023��ƫ�����·��ӵ�����Ϊ60g |
| D��6 gƫ�����º���NA��ƫ�����·��� |
���з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A���ƺ�ˮ��Ӧ���������������������� Na+H2O�TNa++OH-+H2�� |
| B��Cl2��KBr��Һ��Ӧ����������������Cl2+2Br-�T2Cl-+Br2 |
| C��������NaOH��Һ��Ӧ Cl2+OH-�TCl-+ClO-+H2O |
| D��������ʯ��ʯ��Ӧ CO32-+2H+�TCO2��+H2O |
��пƬ��ͭƬΪ��������ϡ����Ϊ�������Һ���ԭ��أ���������ͨ��2mol����ʱ������˵����ȷ���ǣ�������
| A��пƬ�ܽ���1mol����ʱ��ͭƬ��������1mol H2 |
| B���������ܽ�����������ʵ������ |
| C��пƬ�ܽ���31g��ͭƬ��������1g H2 |
| D��пƬ�ܽ���1mol������������1mol |
�ں���FeCl3��FeCl2��AlCl3��NaCl�Ļ����Һ�У�����������Na2O2���壬�����ַ�Ӧ���ټ���������ᣬ��Һ��������Ŀ�ޱ仯���ǣ�������
| A��Na+ |
| B��Al3+ |
| C��Fe2+ |
| D��Fe3+ |
 ��ͼ������ͼƬ�еĻ�ѧʽ���Ƶ������壮
��ͼ������ͼƬ�еĻ�ѧʽ���Ƶ������壮