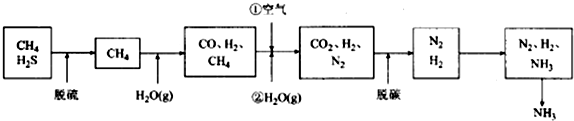
��Ŀ����
3�� ijѧ����0.1000mol•L-1��NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
ijѧ����0.1000mol•L-1��NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����A����ȡ25.0mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�NaOH��Һ��ϴ�ζ���2��3��
C����ʢ�б�עNaOH��Һ�ĵζ��̶ܹ��ã���ѹ������ʹ�ζ��ܼ��������Һ
D��ȡ��NaOH��Һע���ʽ�ζ��ܵ���0���̶�����2��3cm
E������Һ��ֵ��0����0���̶����£����¶���
F������ƿ���ڵζ��ܵ����棬���еζ��������յ㣬�����µζ���Һ��Ķ���
�ش��������⣺
��1����ͼ��������ʽ�ζ��ܵļף�ѡ��ס������ҡ�����
��2����ȷ���������˳����BDCEAF������ĸ��ţ���
��3������B��������Ŀ���Ƿ�ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
��4���жϵ���ζ��յ��ʵ����������Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��5��������Щ������ʹ�ⶨ���ƫ��AC������ţ���
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B��������ƿʱ������ƿ����Һ����
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��6����ƽ��ʵ�����Σ���¼���������±�
| �ζ����� | ������Һ�������/mL�� | ��NaOH��Һ����� | |
| �ζ�ǰ������/mL�� | �ζ��������/mL�� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 0.00 | 22.99 |
| 3 | 25.00 | 0.20 | 20.19 |
���� ��1�����ݵζ��ܵ��ص������
��2�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
��3���ñ�NaOH��Һ��ϴ�ζ��ܣ���ֹ������
��4������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��5������c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϲ��������������������Ӱ�죻
��6�����жϵζ����ݵ���Ч�ԣ������Һ��ƽ�������Ȼ����ݹ�ϵʽHCl��NaOH������������Ũ�ȣ�
��� �⣺��1����ʽ�ζ����¶��Dz�����������ʽ�ζ����¶�����Ƥ��
�ʴ�Ϊ���ף�
��2���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�����������ȷ��˳��Ϊ��BDCEAF��
�ʴ�Ϊ��BDCEAF��
��3���ñ�NaOH��Һ��ϴ�ζ���2��3�Σ���ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
�ʴ�Ϊ����ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
��4����ʵ������NaOH�ζ�������Һ���÷�̪��ָʾ���������յ�ʱ�������ǵ���Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��5��A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ�����ҺŨ�Ȼ��С�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ߣ���A��ȷ��
B��������ƿʱ������ƿ����Һ����������Һ�����ʵ���ƫС�����V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ͣ���B����
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ߣ���C��ȷ��
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ��������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ͣ���D����
��ѡAC��
��6�����εζ����ı�Һ������ֱ�Ϊ��20.01mL��22.99mL��19.99mL���ڶ��εζ�����������Ӧ�������������������ĵı�Һ��ƽ�����Ϊ
20.00mL��
HCl��NaOH
1 1
C��HCl����25.00mL 0.1000mol•L-1��20.00mL��
C��HCl��=$\frac{0.1000mol•{L}^{-1}��20mL}{25mL}$=0.0800mol•L-1��
�ʴ�Ϊ��0.0800mol•L-1��
���� ������Ҫ����������к͵ζ��IJ������衢�ζ��ܵ�ʹ�á����������Ѷ��еȣ������к͵ζ���ԭ���ǽ���Ĺؼ���

| A�� | H2SO4 | B�� | KOH | C�� | FeCl3 | D�� | Ba ��NO3��2 |
| A�� | NH4N03�����ӻ����KN03�ǹ��ۻ����� | |
| B�� | NH4N03�ǹ��ۻ����KN03�����ӻ����� | |
| C�� | NH4N03��KN03�������ӻ����� | |
| D�� | NH4N03��KN03���ǹ��ۻ����� |
| A�� | �ӽ����ƣ���������������Ҵ� | |
| B�� | ��ˮ�����ܵ����Ҵ� | |
| C�� | ��ȼ������ȼ�յ����Ҵ� | |
| D�� | ��Ũ�����Ϲ�����170�棬����ϩ���������Ҵ� |
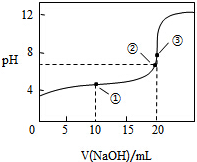 �����£���0.1000mol•L-1 NaOH��Һ�ζ�20.00mL0.1000mol•L-1 CH3COOH��Һ���õζ�������ͼ������˵������ȷ���ǣ�������
�����£���0.1000mol•L-1 NaOH��Һ�ζ�20.00mL0.1000mol•L-1 CH3COOH��Һ���õζ�������ͼ������˵������ȷ���ǣ�������| A�� | �����Һ�Լ��Ե�ԭ���� CH3COO-+H2O�TCH3COOH+OH- | |
| B�� | ���ʱ��Һ��c��Na+������c��CH3COO-�� | |
| C�� | �����Һ�� c��CH3COOH��+c��H+����c��CH3COO-��+c��OH-�� | |
| D�� | ����μ���NaOH��Һ��40mL�Ĺ����У�ˮ�ĵ���̶���������С |
| A�� | pH=3�Ķ�Ԫ����H2R��Һ��p=11��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ�У�2c��R2-��+c��HR-��=��Na+�� | |
| B�� | ��0.3mol•L-1HY��Һ��0.3mol•L-1NaOH��Һ�������Ϻ���Һ��pH=9����c��OH-��-c��HY��=��H+��=1��10-9mol•L-1 | |
| C�� | 0.2mol•L-1HCl��Һ������0.05mol•L-1Ba��OH��2��Һ��Ϻ���Һ��pH=1 | |
| D�� | 0.1mol•L-1Na2S��0.1mol•L-1NaHS�������ϣ�3c��Na+��-2c��HS-��=2c��S2-��+2c��H2S�� |
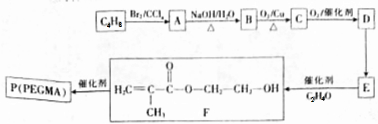
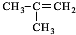 ��
��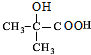 $��_{��}^{Ũ����}$
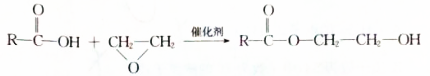
$��_{��}^{Ũ����}$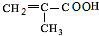 +H2O���÷�Ӧ�ķ�Ӧ��������ȥ��Ӧ��
+H2O���÷�Ӧ�ķ�Ӧ��������ȥ��Ӧ��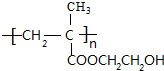 ��
��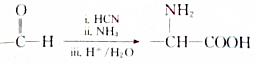 �������B�ϳ�
�������B�ϳ�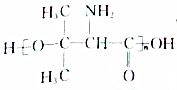 ��·�ߣ��ϳ�·������ͼ��ο�������ʽ��
��·�ߣ��ϳ�·������ͼ��ο�������ʽ��
 ��
��