��Ŀ����
6��ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�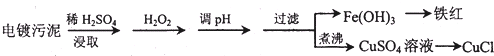
| ������ | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 2.3 | 7.6 | 4.4 |
| ��ȫ������pH | 3.2 | 9.7 | 6.4 |
��2����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ2CuSO4+2NaCl+Na2SO3+H2O=2CuCl��+2Na2SO4+H2SO4��
��3��CuCl��Ʒ��CuCl��������������96.50%Ϊ���Һϸ������ȡ���Ʊ���CuCl��Ʒ0.2500g����һ������0.5mol/LFeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol/L��Ce��SO4��2��Һ�ζ�������ζ��յ�ʱ����Ce��SO4��2��Һ24.60mL���йصĻ�ѧ��ӦΪ��Fe3++CuCl�TFe2++Cu2++Cl-��Ce4++Fe2+�TFe3++Ce3+��ͨ������˵����CuCl��Ʒ���ϣ�����ϡ������ϡ������ұ���
��4��25��ʱ��Ksp[Fe��OH]3]=4.0��10-38��Fe3+����ˮ�ⷴӦFe3++3H2O?Fe��OH��3+3H+���÷�Ӧ��ƽ�ⳣ��Ϊ2.5��10-5��
���� ��1������˫��ˮ�����ʷ�����˫��ˮ�������ԣ���������ԭ�Ե����ʣ����ݹ��˲�����ԭ����ʹ�õ��������з�����ɣ�
��2��CuSO4��+2�۵�ͭ����������������Na2SO3��+4�۵���
��3�����ݹ�ϵʽ����n��CuCl��������������Ʒ��m��CuCl��������0.2500g�ϸ��CuCl�к���CuCl�����������бȽ��жϣ�
��4������ƽ�ⳣ���ĸ����Ϸ�Ӧд������ʽ����ƽ��Ũ�Ƚ��
��� �⣺��1����������к���ͭ�����Ƚ���������������Ҫ���ܽ�ͭ�����Ƚ��������˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+���л�ԭ�ԣ���������H2O2��Fe2+�ܱ�˫��ˮ����Ϊ�����ӣ����ڵ���pHֵ��Cu2+���룬��pH�����м�����Լ�����Ǽ���ij�����ʳ�ȥ��Һ�е����Ҳ������µ����ʣ�����Ҫ��������ͭ����������ͭ��̼��ͭ������Һ��pHֵ4.4ʱ��ͭ���ӿ�ʼ���ֳ���������Һ��pHֵΪ3.2ʱ�����������ӳ�����ȫ��ͭ����δ��������������Ҫʹ���������Ӻ�ͭ���ӷ��룬���˲����õ�������������̨��©�����ձ����������ȣ��������ڲ��������У�©�����ձ�����������
�ʴ�Ϊ����Fe2+������Fe3+�����ڵ���pHֵ��Cu2+���룻CuO����Cu��OH��2����CuCO3��©�����ձ�����������
��2��CuSO4��+2�۵�ͭ�ܰ�Na2SO3��+4�۵���������+6�۵�����CuSO4��Һ�м���һ������NaCl��Na2SO3ʱ���ɰ�ɫ��CuCl��������Ӧ�Ļ�ѧ����ʽΪ��2CuSO4+2NaCl+Na2SO3+H2O=2CuCl��+2Na2SO4+H2SO4��
�ʴ�Ϊ��2CuSO4+2NaCl+Na2SO3+H2O=2CuCl��+2Na2SO4+H2SO4��
��3������Ʒ��CuCl������Ϊx���йصĻ�ѧ��ӦΪ��Fe3++CuCl=Fe2++Cu2++Cl-��Ce4++Fe2+=Fe3++Ce3+����
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n��CuCl�� 24.60��10-3L��0.1000 mol/L
���� n��CuCl��=24.60��10-3L��0.1000 mol/L=2.46��10-3mol��
���Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol=0.2448g��
0.2500g�ϸ��CuCl�к���CuCl������0.2500g��96.5%=0.2413g��С��0.2448g�����Ը���Ʒ��CuCl�������������ϱ���
�ʴ�Ϊ�����ϣ�
��4��Ksp[Fe��OH��3]=c��Fe3+����c3��OH-��=4.0��10-38��c��H+��=$\frac{10{\;}^{-14}}{c��OH{\;}^{-}��}$����ӦFe3++3H2O?Fe��OH��3+3H+��ƽ�ⳣ��K=$\frac{c{\;}^{3}��H{\;}^{+}��}{c��Fe{\;}^{3+}��}$�T$\frac{10{\;}^{-42}}{c��Fe{\;}^{3+}��•c{\;}^{3}��OH{\;}^{-}��}$=2.5��10-5��
�ʴ�Ϊ��2.5��10-5��
���� ������һ��ʵ��̽���⣬�ܽϺõĿ���ѧ�������ͽ������������������˳������ʷ���ķ������ζ�����ȣ�ע��ʵ�鷽�������ԭ���Ͳ������ù�ϵʽ���㣬�������е�֪ʶ������ʼ䷴Ӧ��ʵ������������ʳɷֵ��ƶϣ��ǽ��Ĺؼ���ƽʱע������ʵ�Ļ���֪ʶ�����Ӧ��֪ʶ��������������������Ŀ�Ѷ��еȣ�

| A�� | ������̼�͵��� | B�� | ������̼�Ͷ������� | ||
| C�� | ������̼������ | D�� | ���������� |
| A�� | �о���ѧ��Ӧ�е������仯��������ͨ���ı䷴Ӧ������ʹͬһ��Ӧ������ͬ�������ʶ��ų�������ȣ����ѧ�ܵ������� | |
| B�� | �κη��ȵĻ�ѧ��Ӧ������������ԭ���ֱ�Ӱѻ�ѧ��ת��Ϊ���� | |
| C�� | �о���ѧ��Ӧ�����뻯ѧƽ�⣬������ָ��ʵ�������дﵽ���࣬�죬�ã�ʡ��������Ч�� | |
| D�� | ʹ�ô����������Լӿ컯ѧ��Ӧ���ʣ���������߷�Ӧ���ת���ʣ������ʵ�������н���ʹ�ô��� |
| A�� | ����Һ�ķ�ɢ�ʿ��ù��˵ķ����ӷ�ɢ���з������ | |
| B�� | ��ɢ������ֱ��Ϊ1-100���ķ�ɢϵ�ǽ��� | |
| C�� | ��ͬ�¶���ͬһ�����ʵı�����ҺҪ�Ȳ�������Һ��Ũ�ȴ�һЩ | |
| D�� | �κ�������ˮ���ܽⶼ��һ�����ܽ�� |
| A�� | ������Һ��ȥ������ | |
| B�� | ��NaHCO3��Һ��Al2��SO4��3��Һ����������ĭ���� | |
| C�� | TiCl4���ڴ���ˮ�����Ʊ�TiO2 | |
| D�� | ��п����ϡ���ᷴӦ��ȡ����ʱ�μ�����CuSO4��Һ |