��Ŀ����
9��ϩ��A��һ�������¿�������Ŀ�ͼ���з�Ӧ��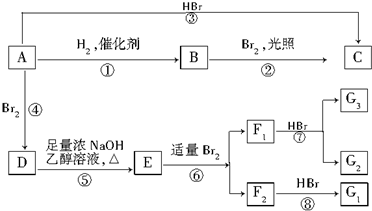
��D��
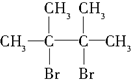 ����F1��F2��Ϊͬ���칹�壩��G1��G2��Ϊͬ���칹�壩
����F1��F2��Ϊͬ���칹�壩��G1��G2��Ϊͬ���칹�壩��֪��CH3CH2CH2CH2CH2CH2Br$��_{��}^{ŨNaOH���Ҵ���Һ}$CH3CH2CH2CH2CH�TCH2���Իش��������⣺
��1��A�Ľṹ��ʽ����CH3��2C=C��CH3��2��
��2����ͼ������ȡ����Ӧ���ǣ������ִ��ţ��ڣ�
��3����ͼ�Т١��ۡ������ڼӳɷ�Ӧ��
��4��G1�Ľṹ��ʽ��
 ��
��
���� ��D��֪AΪ��CH3��2C=C��CH3��2����BΪ��CH3��2CH-CH��CH3��2���ɷ�Ӧ�ۿ�֪CΪ ��D��NaOH����Һ�м��������·�����ȥ��Ӧ��EΪCH2=C��CH3��-C��CH3��=CH2��CH2=C��CH3��C��CH3��=CH2�����巢��1��2�ӳɻ�1��4�ӳɣ�F1���廯������ֲ����F2���廯���һ�ֲ����F1��F2��������G1����F1ΪCH2BrCBr��CH3��C��CH3��=CH2��F2ΪCH2BrC��CH3��=C��CH3��CH2Br��G1Ϊ
��D��NaOH����Һ�м��������·�����ȥ��Ӧ��EΪCH2=C��CH3��-C��CH3��=CH2��CH2=C��CH3��C��CH3��=CH2�����巢��1��2�ӳɻ�1��4�ӳɣ�F1���廯������ֲ����F2���廯���һ�ֲ����F1��F2��������G1����F1ΪCH2BrCBr��CH3��C��CH3��=CH2��F2ΪCH2BrC��CH3��=C��CH3��CH2Br��G1Ϊ ��G2ΪCH2BrCBr��CH3��CBr��CH3��CH3���ݴ˷������
��G2ΪCH2BrCBr��CH3��CBr��CH3��CH3���ݴ˷������
��� �⣺��D��֪AΪ��CH3��2C=C��CH3��2����BΪ��CH3��2CH-CH��CH3��2���ɷ�Ӧ�ۿ�֪CΪ ��D��NaOH����Һ�м��������·�����ȥ��Ӧ��EΪCH2=C��CH3��-C��CH3��=CH2��CH2=C��CH3��C��CH3��=CH2�����巢��1��2�ӳɻ�1��4�ӳɣ�F1���廯������ֲ����F2���廯���һ�ֲ����F1��F2��������G1����F1ΪCH2BrCBr��CH3��C��CH3��=CH2��F2ΪCH2BrC��CH3��=C��CH3��CH2Br��G1Ϊ
��D��NaOH����Һ�м��������·�����ȥ��Ӧ��EΪCH2=C��CH3��-C��CH3��=CH2��CH2=C��CH3��C��CH3��=CH2�����巢��1��2�ӳɻ�1��4�ӳɣ�F1���廯������ֲ����F2���廯���һ�ֲ����F1��F2��������G1����F1ΪCH2BrCBr��CH3��C��CH3��=CH2��F2ΪCH2BrC��CH3��=C��CH3��CH2Br��G1Ϊ ��G2ΪCH2BrCBr��CH3��CBr��CH3��CH3��
��G2ΪCH2BrCBr��CH3��CBr��CH3��CH3��
��1��ͨ�����Ϸ���֪��A�ṹ��ʽΪ��CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����Ӧ��Ϊ�ӳɷ�Ӧ����Ϊȡ����Ӧ����Ϊ�ӳɷ�Ӧ����Ϊ�ӳɷ�Ӧ����Ϊ��ȥ��Ӧ����Ϊ�ӳɷ�Ӧ����Ϊ�ӳɷ�Ӧ����Ϊ�ӳɷ�Ӧ��
�ʴ�Ϊ���ڣ�
��3����ͼ�Т١��ۡ������ڼӳɷ�Ӧ���ʴ�Ϊ���ӳɣ�
��4��G1�Ľṹ��ʽ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ���������ȷ�����л�������ż������ʡ���Ӧ��������Ӧ�����ǽⱾ��ؼ�����D�ṹ��ʽΪͻ�ƿڽ����ƶϣ���Ŀ�ѶȲ���

| A�� | ̼���ƣ����壩 | B�� | ˮ | C�� | �������Һ | D�� | ����泥����壩 |
 �������йظ�����������ȷ���ǣ�������
�������йظ�����������ȷ���ǣ�������| A�� | ����������Ʒ�����Ӧ | |
| B�� | ����ʹ���Ը��������Һ��ɫ | |
| C�� | ��Ũ������£��������ᷢ����Ӧ | |
| D�� | ����������������Ȼ�̼��Һ�����ӳɷ�Ӧ |
| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
��1������Ԫ���д���VA����У��ܢߣ��ñ�ű�ʾ��
��2�����ڵ�2���ڵ�Ԫ���У��٢ۢߢ౻��Ϊ��������һ������п϶����б��Т٣����Ͼ��ñ�ű�ʾ��
��3��д���ڡ��ݵ�Ԫ�ط��ţ�Mg��Cl��
��4������Ԫ���н�������ǿ���ǣ�Na����Ԫ�ط��ű�ʾ��
| A�� | CH3CH2CH3 | B�� | CH3-O-CH3 | C�� |  | D�� | 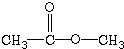 |
��Ҫ�ɷ֣�Al2O3���ʣ�Fe2O3��SiO2�����������Ҳ����뻯ѧ��Ӧ������Ϊ�ⶨ����Ʒ��Al�ĺ�����ij��ѧ��ȤС���������������������
���������ϡ���������ӿ�ʼ�����ͳ�����ȫʱ��Һ��pH�����
| Al3+ | Fe3+ | AlO2- | SiO32- | |
| ��ʼ����ʱ | 3.4 | 1.9 | 10.6 | 7.3 |
| ������ȫʱ | 4.7 | 3.2 | 9.3 | 5.3 |
��������

��1���ڷ�Ӧ�����ӷ���ʽΪOH-+CO2=CO32-+H2O��2AlO2-+CO2+3H2O=CO32-+2Al��OH��3����CO2���ɹ�����ԭ���Ƿ�ֹSiO32-��������
��2�����ڲ�����м���������������pH������Ҳ�ɵõ�d�ij�����д������d�����Ļ�ѧ����ʽAlO2-+CH3COOCH2CH3+2H2O$\frac{\underline{\;\;��\;\;}}{\;}$CH3COO-+Al��OH��3��+CH3CH2OH��
| A�� | O2��O3 | B�� | 35Cl��37Cl | C�� | 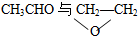 | D�� | CH4��C2H6 |
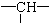 ������Ϊ��ԭ�ӣ�����ԭ�ӵĸ���Ϊ��������
������Ϊ��ԭ�ӣ�����ԭ�ӵĸ���Ϊ��������| A�� | 2y+3x-x | B�� | z+2-x | C�� | 2y+z-x | D�� | z+2y+2-x |