��Ŀ����
9�� ��֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100�������֮�ȣ���ʹ�ã���ش��������⣺
��֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100�������֮�ȣ���ʹ�ã���ش��������⣺��1���á�84����Һ�������ʵ���Ũ��ԼΪ4.0L/mol��������λ��Ч���֣�
��2����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480mL��NaClO��������Ϊ25%������Һ�������õ�����ƿ��������˵����ȷ����C������ţ���
A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
D����Ҫ����NaClO���������Ϊ143.0g
��3����ʵ���������������������Һ�����ʵ���Ũ���ǣ����á�ƫ�͡�����ƫ�ߣ������䡱�ش�
����ʱ���ӿ̶���ƫ�ߣ�
����ʱˮ���ý�ͷ�ι�����ƫ�ͣ�
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%���ܶ�Ϊ1.84g•cm-3����Ũ��������2000mL 2.3mol•L-1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H+�����ʵ���Ũ��Ϊ4.6mol•L-1��
������Ũ��������Ϊ250 mL��
���� ��1������C=$\frac{1000�Ѧ�}{M}$������Һ�����ʵ���Ũ�ȣ�
��2��A����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
B������ʱ������Ҫ��������ˮ��
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС������C=$\frac{n}{V}$���з�����
D������m=CVM������Ҫ���ʵ�������
��3�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��4���ٸ�������Ļ�ѧʽ�����2.3mol•L-1��ϡ������������Ũ�ȣ�
�ڸ���c=$\frac{1000�Ѧ�}{M}$�����Ũ�����Ũ�ȣ����ƹ�������������ʵ������䣬����V=$\frac{n}{c}$�������ҪŨ����������
��� �⣺��1���á�84����Һ�������ʵ���Ũ��C=$\frac{1000��1.19��25%}{74.5}$=4.0mol/L��
�ʴ�Ϊ��4.0L/mol��
��2��A������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ����Ͳ����ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����Ҫ���ǣ�Բ����ƿ�ͷ�Һ©��������Ҫ����������ͷ�ιܣ���A����
B������ʱ������Ҫ��������ˮ����������ƿʹ��ǰ����Ҫ���У���B����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС������C=$\frac{n}{V}$��֪��ҺŨ��ƫ�ͣ���Cѡ��
D������480mL��NaClO��������Ϊ25%������Һ�������õ�����ƿ�������ʵ���Ũ��Ϊ4.0L/mol����Ҫѡ��500mL����ƿ����Ҫ���ʵ�����m=4.0mol/L��74.5g/mol��0.5L=149g����D����
��ѡ��C��
��3������ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
����ʱˮ���ý�ͷ�ι��������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��4�����������Ƶ�ϡ�����Ũ��Ϊ2.3mol/L������Һ��H+�����ʵ���Ũ��Ϊ��c��H+��=2c��H2SO4��=2.3mol/L��2=4.6mol/L��
�ʴ�Ϊ��4.6��
��98%���ܶ�Ϊ1.84g•cm-3����Ũ����Ũ��Ϊ��C=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L��
����2000mL 2.3mol•L-1��ϡ���ᣬ����Ũ��������Ϊ��$\frac{2.3mol/L��2L}{18.4mol/L}$=0.25L=250mL��
�ʴ�Ϊ��250��
���� ���⿼����һ�����ʵ���Ũ����Һ�����Ƽ��й����ʵ���Ũ�ȼ��㣬��ȷ����ԭ�������������ǽ���ؼ���ע�������������ʵ������������������Ĺ�ϵ������������ѧ���Ļ�ѧ����������

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�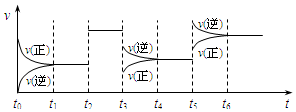 ij�ܱ������з������·�Ӧ��X��g��+3Y��g��?2Z��g������H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����
ij�ܱ������з������·�Ӧ��X��g��+3Y��g��?2Z��g������H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ������������
| A�� | t2ʱ�����˴��� | B�� | t3ʱ�������¶� | ||
| C�� | t5ʱ�������¶� | D�� | t4��t5ʱ����ת����һ����� |
| A�� | b��c��a | B�� | b��a��c | C�� | c��b��a | D�� | a��b��c |
��1����һ���¶��£��ڹ̶�������ܱ������н��п��淴Ӧ��N2+3H2?2NH3 �ÿ��淴Ӧ�ﵽƽ��ı�־��BCE��
A��3v��H2����=2v��NH3����
B����λʱ������m mol N2��ͬʱ����3m mol H2
C�������ڵ���ѹǿ������ʱ����仯
D�����������ܶȲ�����ʱ����仯
E��a mol N��N�����ѵ�ͬʱ����6a mol N-H������
F��N2��H2��NH3�ķ�����֮��Ϊ1��3��2
��2��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɰ��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1mol N2��3mol H2��������ʴ�����������Ժ��Բ��ƣ�����һ���¶�ѹǿ�¿�ʼ��Ӧ������ѹ���Ƽ��������ѹǿ�ı仯�����
| ��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
| ѹǿ/MPa | 16.80 | 14.78 | 13.86 | 13.27 | 12.85 | 12.60 | 12.60 |
���¶���ƽ�ⳣ��K=2.37��