��Ŀ����
13�����������۾�����W����֬X�ĺϳ�·����ͼ��
��֪��

��1��������������ܶ���29���ܷ���������Ӧ���Ľṹ��ʽΪCH3CH2CHO��
��2��A �к��еĹ�����������ȩ����̼̼˫����
��3��C��W ��Ӧ�Ļ�ѧ����ʽ��n CH2=C��CH3��COOCH2CH2OH$\stackrel{����}{��}$
 ��
����4�����й�������C ��˵����ȷ��ac
a���ܷ����ӳɡ��Ӿۡ�ȡ������ȥ��Ӧ b������˳���칹��
c����ʹ���Ը��������Һ��ɫ d��������������ͬ���칹��
��5��B �ж���ͬ���칹�壮�������Һ���̼̼˫����ͬ���칹�干��5�֣�������˳���칹����ͬ����д�������ܷ���������Ӧ���Һ��м�������ͬ���칹��Ľṹ��ʽ��HCOOCH=CHCH3��HCOOC��CH3��=CH2��
��6��Ϊȷ��ij�����廯����F�Ľṹ������������ʵ�飺
�پ������Dzⶨ�����л������Է�������Ϊ110
��ȷ��ȡ���л�����Ʒ5.50g�����ȼ�պ����ɱ�״����6.72LCO2��2.70gH2O
����˴Ź�����ͼ��ʾ��F��2�����շ壬ǿ��Ϊ2��1����F�Ľṹ��ʽΪ
 ��
����7��E ������������˴Ź�����ͼ����4 �����շ壮д������E�Ļ�ѧ����ʽ
 ����Ӧ�����Ǽӳɷ�Ӧ��
����Ӧ�����Ǽӳɷ�Ӧ��
���� ��W�Ľṹ���ƿ�֪CΪCH2=C��CH3��COOCH2CH2OH��B���Ҷ�������������Ӧ����C����BΪCH2=C��CH3��COOH��A��B����������Ӧ����AΪCH2=C��CH3��CHO��������������ܶ���29����Է�������Ϊ58���ܷ���������Ӧ������ȩ��������HCHO��Ӧ����ˮ����A�����ΪCH3CH2CHO��A��D�����ӳɷ�Ӧ����DΪCH3CH��CH3��CH2OH��D������ȥ��Ӧ����CH2=C��CH3��2����6���з����廯����F����Է�������Ϊ110��5.50gF�����ʵ���Ϊ$\frac{5.5g}{110g/mol}$=0.05mol�����ȼ�պ����ɱ�״����6.72L CO2��2.70g H2O��������̼Ϊ0.3mol��ˮΪ$\frac{2.7g}{18g/mol}$=0.15mol���������Cԭ����ĿΪ6��Hԭ����ĿΪ6����ԭ����ĿΪ$\frac{110-12��6-6}{16}$=2����F�ķ���ʽΪC7H7O2��F��2�����շ壬ǿ��Ϊ2��1����F�Ľṹ��ʽΪ ��F���ȩ�������۷�Ӧ������֬X��CH2=C��CH3��2��F��Ӧ�õ�E����E������������˴Ź�����ͼ����4�����շ壬��CH2=C��CH3��2��F�����ӳɷ�Ӧ����E�Ľṹ��ʽΪ
��F���ȩ�������۷�Ӧ������֬X��CH2=C��CH3��2��F��Ӧ�õ�E����E������������˴Ź�����ͼ����4�����շ壬��CH2=C��CH3��2��F�����ӳɷ�Ӧ����E�Ľṹ��ʽΪ ��
��
��� �⣺��W�Ľṹ���ƿ�֪CΪCH2=C��CH3��COOCH2CH2OH��B���Ҷ�������������Ӧ����C����BΪCH2=C��CH3��COOH��A��B����������Ӧ����AΪCH2=C��CH3��CHO��������������ܶ���29����Է�������Ϊ58���ܷ���������Ӧ������ȩ��������HCHO��Ӧ����ˮ����A�����ΪCH3CH2CHO��A��D�����ӳɷ�Ӧ����DΪCH3CH��CH3��CH2OH��D������ȥ��Ӧ����CH2=C��CH3��2����6���з����廯����F����Է�������Ϊ110��5.50gF�����ʵ���Ϊ$\frac{5.5g}{110g/mol}$=0.05mol�����ȼ�պ����ɱ�״����6.72L CO2��2.70g H2O��������̼Ϊ0.3mol��ˮΪ$\frac{2.7g}{18g/mol}$=0.15mol���������Cԭ����ĿΪ6��Hԭ����ĿΪ6����ԭ����ĿΪ$\frac{110-12��6-6}{16}$=2����F�ķ���ʽΪC7H7O2��F��2�����շ壬ǿ��Ϊ2��1����F�Ľṹ��ʽΪ ��F���ȩ�������۷�Ӧ������֬X��CH2=C��CH3��2��F��Ӧ�õ�E����E������������˴Ź�����ͼ����4�����շ壬��CH2=C��CH3��2��F�����ӳɷ�Ӧ����E�Ľṹ��ʽΪ
��F���ȩ�������۷�Ӧ������֬X��CH2=C��CH3��2��F��Ӧ�õ�E����E������������˴Ź�����ͼ����4�����շ壬��CH2=C��CH3��2��F�����ӳɷ�Ӧ����E�Ľṹ��ʽΪ ��
��
��1��������������֪���Ľṹ��ʽΪCH3CH2CHO���ʴ�Ϊ��CH3CH2CHO��
��2��AΪCH2=C��CH3��CHO�����еĹ�����������ȩ����̼̼˫�����ʴ�Ϊ��ȩ����̼̼˫����
��3��C��W ��Ӧ��ѧ����ʽ��n CH2=C��CH3��COOCH2CH2OH$\stackrel{����}{��}$ ��
��
�ʴ�Ϊ��n CH2=C��CH3��COOCH2CH2OH$\stackrel{����}{��}$ ��
��
��4������C�Ľṹ��ʽΪ��CH2=C��CH3��COOCH2CH2OH��
a������̼̼˫�����ܷ����ӳɷ�Ӧ���Ӿ۷�Ӧ�������ǻ������Է���ȡ����Ӧ����ȥ��Ӧ����a��ȷ��
b������һ��������̼ԭ������2��Hԭ�ӣ�û��˳���칹�壬��b����
c������̼̼˫�����ǻ�����ʹ���Ը��������Һ��ɫ����c��ȷ��
d���������������Դ���������ͬ���칹�壬��d����
��ѡ��ac��
��5��BΪCH2=C��CH3��COOH���������Һ���̼̼˫����ͬ���칹���У�HCOOCH=CHCH3��HCOOCH2CH=CH2��HCOOC��CH3��=CH2��CH3COOCH=CH2��CH2=CHCOOCH3����5�֣������ܷ���������Ӧ���Һ��м�������ͬ���칹��Ľṹ��ʽΪ��HCOOCH=CHCH3��HCOOC��CH3��=CH2��
�ʴ�Ϊ��5��HCOOCH=CHCH3��HCOOC��CH3��=CH2��
��6��������������֪����F�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��7������E�Ļ�ѧ����ʽΪ �����ڼӳɷ�Ӧ��
�����ڼӳɷ�Ӧ��
�ʴ�Ϊ�� ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
���� ���⿼���л�����ƶϣ��������ת�����л���ṹ�뷴Ӧ���������ƶϣ��������չ����ŵ�������ת�����ϺõĿ���ѧ����������������֪ʶǨ��Ӧ�ã���Ŀ�Ѷ��еȣ�

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�| A�� | ������ȡ��ܶȲ�ͬ��N2��C2H4 | B�� | �����ȵ�CO��N2 | ||
| C�� | ���¡��������O2��N2 | D�� | ��ѹ���������N2��CH4 |
| A�� | �ȷ� | B�� | �屽 | C�� | 2-��-2-������ | D�� | �Ҵ� |
| A�� | �ף��ң������� | B�� | �ң��ף������� | C�� | ���������ң��� | D�� | �ң��ף������� |
| A�� | ԭ�Ӱ뾶��Na��Mg��Al | B�� | ����ǿ����NaOH��Mg��OH��2��Ca��OH��2 | ||
| C�� | ���ȶ��ԣ�HCl��PH3��AsH3 | D�� | ����ǿ����H4SiO4��H3PO4��HNO3 |
| A�� | Al2O3��ϡ���� | B�� | Al2O3��ˮ | C�� | Al2��SO4��3��NaOH | D�� | AlCl3�Ͱ�ˮ |
| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| B�� | 1L 0.1mol/L��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23 g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | �ܱ�������2mol NO��1molO2��ַ�Ӧ������ķ�����Ϊ2NA |
 ����C���ʱ�����һ±����ֻ�����֣�
����C���ʱ�����һ±����ֻ�����֣�
 ��F�ķ���ʽC9H10��
��F�ķ���ʽC9H10�� ��
��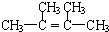 ��
�� ��
��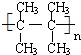 �ڴ��������£���H2�ӳɺ�IJ�������Ϊ2��3-�������飮
�ڴ��������£���H2�ӳɺ�IJ�������Ϊ2��3-�������飮