��Ŀ����
8�����и���ʵ������ó��Ľ�����ȷ���ǣ�������| ѡ�� | ʵ����� | ���� | ���� |
| A | ��FeCl3ʴ��Һ�м����������ۡ��� | �õ�������Һ | ʴ��Һ��һ������Cu2+ |
| B | ��Fe��NO3��2��Ʒ����ϡH2SO4���μ�KSCN��Һ | ��Һ��� | ϡ����������Fe2+ |
| C | KBrO3��Һ�м�����������Ȼ��ͨ������Cl2����������� | �л�����ֳ�ɫ | �����ԣ�Cl2��Br2 |
| D | ��������Һ������Cu��OH��2��ϼ��� | ����ש��ɫ���� | �����Ƿ����к���ȩ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A���������۲��㣻
B�����������£�NO3-�ܰ�Fe2+����ΪFe3+��
C��KBrO3��Һ�м�����������Ȼ��ͨ������Cl2���л���ʳ�ɫ��˵���÷�Ӧ����Br2���ɣ�BrԪ�ػ��ϼ���+5�۱�Ϊ0�ۣ�����KBrO3������������Cl2�ǻ�ԭ����
D�������Ƿ����к���ȩ�����ܹ�������������ͭ��Һ��Ӧ����ש��ɫ��������ͭ������
��� �⣺A���������۲��㣬ͭ����δ��Ӧ����A����
B����Fe��NO��2��Ʒ����ϡH2SO4�����������£�NO3-�ܰ�Fe2+����ΪFe3+���μ�KSCN��Һ��죬����˵��Fe��NO3��2�����Ѿ����ʣ���B����
C��KBrO3��Һ�м�����������Ȼ��ͨ������Cl2���л���ʳ�ɫ��˵���÷�Ӧ����Br2���ɣ�BrԪ�ػ��ϼ���+5�۱�Ϊ0�ۣ�����KBrO3������������Cl2�ǻ�ԭ����Br2�ǻ�ԭ������Բ���˵��������Cl2��Br2����C����
D����������Һ������Cu��OH��2��ϼ��ȣ�������ש��ɫ������֤���������Ƿ����к���ȩ������ʵ���ܹ��ﵽʵ��Ŀ�ģ���D��ȷ��
��ѡD��
���� ���⿼��������ʵ�鷽������������ۣ���ȷ�������ʵ���ɡ��ṹ������Ϊ���ؼ�������ʵ�����������ʵ��ԭ���Ŀ��飬ע��װ�õ����ü�ʵ��IJ����ԡ������Է�������Ŀ�ѶȲ���

 ������ϵ�д�
������ϵ�д�| A�� | ���� | B�� | ���� | C�� | �Ȼ�����Һ | D�� | ������Һ |
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
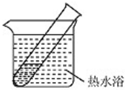 | ʵ��� | 2mL������Һ���� �ν�ŨNaOH��Һ | �����ݲ����� һ��ʱ�����Һ ��ڣ��Թܱ� �������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ����� һ��ʱ�����Һ �����Ա仯 |
a��Ag��NH3��2++2H2O=Ag++2NH3+H2O b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
��1������������Һ�����ҩƷ��AgNO3��Һ��Ũ��ˮ��
��2�������飬ʵ������������NH3����ɫ��������Ag2O��
����ʪ��ĺ�ɫʯ����ֽ����NH3����������������ֽ������
�ڲ���Ag2O��ԭ������NaOH�����£����ȴٽ�NH3•H2O�ֽ⣬�ݳ�NH3����ʹƽ��Ag��NH3��2++2H2O?Ag++2NH3•H2O�����ƶ���c��Ag+������Ag+��OH-��Ӧ2OH-+2Ag+=Ag2O+H2O������ת��ΪAg2O��
��3����ͬѧ�Բ���������ԭ��������裺������NaOH��ԭAg2O��ʵ�鼰������AgNO3��Һ�м������NaOH��Һ�����ֺ�ɫ������ˮԡ���ȣ�δ����������
��4�����¼��裺��NaOH�����£�������NH3����ԭAg2O����ͼ1��ʾװ�ý���ʵ�飮�����������������߿��ڻ�������ʯ�Һ�Ũ��ˮ��ȡNH3��װ�ü�ͼ���г������ԣ���

��5����ͬѧ��Ϊ�ڣ�4����ʵ���л���Ag��NH3��2OH���ɣ��ɴ���������裺��NaOH�����£�������Ag
��NH3��2OHҲ������NH3��ԭAg2O�ķ�Ӧ��������ͼ2��ʵ�飺
���в���Ag2O�ܽ��ڰ�ˮ�У��÷�Ӧ�Ļ�ѧ����ʽ��Ag2O+4 NH3•H2O=2Ag��NH3��2OH+3H2O��
��ʵ����֤ʵ������������ݵ�����������Һ�Ӵ����Թܱ�������������
��6����HNO3��ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ��4HNO3��ϡ��+3Ag�T3AgNO3+NO��+2H2O��
| A�� |  Al��OH��3 | B�� |  C6H12O6 | C�� |  CH3CH2OH | D�� |  NaHCO3 |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
 ��
��