��Ŀ����
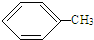
9����Ҫ��д������ת����Ӧ�Ļ�ѧ����ʽ��1���ټױ���2��4��6�������ױ�
 +3HO-NO2$\stackrel{Ũ����}{��}$
+3HO-NO2$\stackrel{Ũ����}{��}$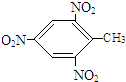 +3H2O��
+3H2O���ڱ�ϩ���۱�ϩ
 ��
����2-��������ϩCH3-CHBr-CH3+NaOH$��_{��}^{��}$CH3-CH=CH2��+NaBr+H2O��
��ϩ����һ������������ʱ������C=C�����ѣ�ת��Ϊȩ��ͪ���磺

��
 ���������������·�����Ӧ����д����Ӧ�ķ���ʽ
���������������·�����Ӧ����д����Ӧ�ķ���ʽ ��
����2��1��3-����ϩ���巢���ӳɷ�Ӧ���ܵõ�3 �ֲ��3��
���� ��1���ټױ���������Ũ����������·���ȡ����Ӧ����2��4��6-�������ױ���
�ڱ�ϩ����̼̼˫���������Ӿ۷�Ӧ���ɾ۱�ϩ��
��2-��������������ƵĴ���Һ�з�����ȥ��Ӧ���ɱ�ϩ���廯�ƺ�ˮ��
��ϩ������ԭ��Ϊ����˫�����м�Ͽ�ֱ�ӽ���ԭ�ӣ�
��2��CH2=CH-CH=CH2����2��C=C�������巢��1��1�ӳ�ʱ���ɷֱ�����CH2Br-CHBrCH=CH2��CH2Br-CH=CH-CH2Br��������ȫ�ӳɰ�1��2��Ӧ����CH2Br-CHBrCHBrCH2Br���Դ˽����⣮
��� �⣺��1���ټױ���������Ũ����������·���ȡ����Ӧ����2��4��6-�������ױ�����ѧ����ʽ�� +3HO-NO2$\stackrel{Ũ����}{��}$
+3HO-NO2$\stackrel{Ũ����}{��}$ +3H2O���ʴ�Ϊ��
+3H2O���ʴ�Ϊ�� +3HO-NO2$\stackrel{Ũ����}{��}$
+3HO-NO2$\stackrel{Ũ����}{��}$ +3H2O��
+3H2O��
�ڱ�ϩ�����Ӿ۷�Ӧ���ɾ۱�ϩ����ѧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2-��������������ƵĴ���Һ�з�����ȥ��Ӧ���ɱ�ϩ���廯�ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3-CHBr-CH3+NaOH$��_{��}^{��}$CH3-CH=CH2��+NaBr+H2O��
�ʴ�Ϊ��CH3-CHBr-CH3+NaOH$��_{��}^{��}$CH3-CH=CH2��+NaBr+H2O��
��ϩ������ԭ��Ϊ����˫�����м�Ͽ�ֱ�ӽ���ԭ�ӣ���Ӧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��2��CH2=CH-CH=CH2����2��C=C���������尴1��1��1��2�ӳɣ�
1��2�ӳɷ���CH2=CH-CH=CH2+Br2��CH2Br-CHBrCH=CH2��
CH2Br-CHBrCH=CH2����Ϊ��3��4-����-1-��ϩ��
1��4�ӳɷ���CH2=CH-CH=CH2+Br2��CH2Br-CH=CH-CH2Br��
CH2Br-CH=CH-CH2Br����Ϊ��1��4-����-2-��ϩ��
��ȫ�ӳɷ���CH2=CH-CH=CH2+2Br2��CH2BrCHBrCHBrCH2Br��
CH2BrCHBrCHBrCH2Br����Ϊ��1��2��3��4-���嶡�飻
�ʴ�Ϊ��3��
���� ���⿼�黯ѧ����ʽ����д����Ϥ��Ӧ��ԭ���Լ�ע����ȷ��д��Ӧ����ʽӦע��������ǽ��Ĺؼ�����Ŀ�ѶȲ���

| A�� | �μ�KI��Һʱ��ת��2 mol e-ʱ���� 1 mol��ɫ���� | |
| B�� | ͨ��SO2����Һ�����ɫ������ SO2��Ư���� | |
| C�� | ͨ��SO2ʱ��SO2��I2��Ӧ��I2�������� | |
| D�� | ����ʵ�������£����ʵ������ԣ�Cu2+��I2��SO2 |
| A�� | ��������ϩ��Ϊͬϵ�� | |
| B�� | 32S��33S��ͬ�ֺ��� | |
| C�� | O2��O3��Ϊͬλ�� | |
| D�� | �Ҵ��Ͷ����ѣ�CH3-O-CH3����Ϊͬ���칹�� |
| A�� | �ǻ��ĵ���ʽ | B�� | CH4���ӵ����ģ�� | ||
| C�� | ��ϩ�����ʽ��ʵ��ʽ�� CH2 | D�� | ����ȩ�ṹ��ʽ  |
| A�� | Ag+ NH3 | B�� | Cu2+ NH3 | C�� | H+ H2O | D�� | Ag+ CH4 |


 ��
�� ��
��