��Ŀ����
9����֪���ᾧ�壨H2C2O4��XH2O��������ˮ�����������Ը��������Һ��ȫ��Ӧ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+8H2O+10CO2��
����������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X���������£�
���÷�����ƽ��ȡ���ᾧ��1.440g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊ0.1000mol•L-1��KMnO4����Һ���еζ������ν�����£�
| ��һ�εζ� | �ڶ��εζ� | �����εζ� | |
| ������Һ�����mL�� | 25.00 | 25.00 | 25.00 |
| ����Һ�����mL�� | 9.99 | 10.01 | 10.00 |
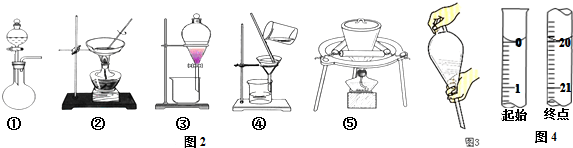
��1���ζ�ʱ��KMnO4����ҺӦ��װ����ʽ������ʽ���ʽ���ζ����У�
��2��������ʵ������У�����Ҫ����������Ʒ�Ǣܢޣ�����ţ���
��100mL����ƿ ���ձ� �۵ζ��ܼ� ��©�� �ݲ����� ��������ƽ
��3������ζ��յ�ı�־�Ǽ������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����30s�ڲ���ɫ��
��4�������������ݼ���X=3��
��5�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵƫ�ߣ��ƫ�ߡ�ƫ�͡���Ӱ�죩��
��6����KMnO4����ҺŨ��ƫ�ͣ���Xֵƫ�ͣ��ƫ�ߡ�ƫ�͡���Ӱ�죩��
���� ��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ��
��3������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊdz�Ϻ�ɫ��
��4���������ѧ����ʽ�����ݣ��Ӷ�����X��ֵ��
��5����6����c�����⣩=$\frac{c������V������}{V�����⣩}$���������������V�������ı仯������
��� �⣺��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧװ����ʽ�ζ����У�
�ʴ�Ϊ����ʽ��
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ�����Բ���Ҫ�������Тܢޣ�
�ʴ�Ϊ���ܢޣ�
��3�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����ɫ30s�ڲ���ɫ��˵���ζ����յ㣬
�ʴ�Ϊ���������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����30s�ڲ���ɫ��
��4�����εζ�����Ч��KMnO4����Һ��ƽ�����Ϊ10.00mL����25.00mL���������Һ���ĵ�KMnO4��
���ʵ���Ϊ0.1000mol•L-1��0.01L��100.00mL���������Һ���ĵ�KMnO4�����ʵ���Ϊ0.1000mol•L-1��0.01L��4����1.440g�����ᾧ�����ĵ�KMnO4�����ʵ���Ϊ0.1000mol•L-1��0.01L��4����2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��֪H2C2O4�����ʵ���Ϊ��0.1000mol•L-1��0.01L��4��$\frac{5}{2}$=0.01mol��0.01molH2C2O4������Ϊ0.01mol��90g/mol=0.9g������1.440gH2C2O4•xH2O��ˮ�����ʵ���Ϊ1.440g-0.9g=0.54g�������ʵ���=$\frac{0.54g}{18g/mol}$=0.03mol����x=3��
�ʴ�Ϊ��3��
��5���ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ������Һ�����С���������ʵ�����С��������С����ˮƫ�࣬Xֵƫ�ߣ�
��Ϊ��ƫ�ߣ�
��6��KMnO4����ҺŨ��ƫ�ͣ����ñ�Һ���ƫ������IJ������ʵ���ƫ�࣬����ƫ��ˮ������ƫС��XƫС��
��Ϊ��ƫ�ͣ�
���� ���⿼����������ԭ�ζ���ʵ����̷�����ʵ�����������ע��ζ��ܵ�ʹ�ú͵ζ����㣬��Ŀ�Ѷ��еȣ�

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�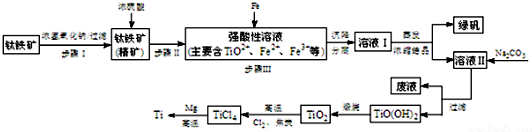
��֪����2H2SO4��Ũ��+FeTiO3=TiOSO4+FeSO4+2H2O
��TiO2+��ˮ�⣬ֻ�ܴ�����ǿ������Һ�У�
��1������I�з�����Ӧ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O��SiO2+2OH-=SiO32-+H2O��
��2��25��ʱ�����ܵ�����γɳ�����pH��ϵ���
| pH | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 | Ti��OH��2 |
| ��ʼ���� | 1.1 | 4.5 | 7 | 1 |
| ��ȫ���� | 2.8 | 6.4 | 9.2 | 2.7 |
a������II������мԭ���ǽ�Fe3+ת��ΪFe2+����ֹFe3+��TiO2+ͬʱ���ɳ�����
b������ҺII�м���Na2CO3��ĩ�������ǵ���PH������TiO��OH��2��Һ����Һ���д������е���������TiO2+��Fe2+��H+��
��3��TiCl4��Ti��Ӧ��õ�Mg��MgCl2��Ti�Ļ����ɲ����������ķ�������õ�Ti��
��д����TiO2��ȡTiCl4�Ļ�ѧ����ʽTiO2+2Cl2+2C$\frac{\underline{\;����\;}}{\;}$TiCl4+2CO��
�����������Ϣ������ȵ��¶��Ը���1412�漴�ɣ�
| TiCl4 | Mg | MgCl2 | Ti | |
| �۵�/�� | -25.0 | 648.8 | 714 | 1667 |
| �е�/�� | 136.4 | 1090 | 1412 | 3287 |
 ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����| ����a | ��̿���ڸ��������»�ԭCuO |
| ����b | �������ǻ�ԭ���Ƶ�Cu��OH��2�Ʊ�Cu2O |
| ����c | ��ⷨ����ӦΪ2Cu+H2O$\frac{\underline{\;���\;}}{\;}$Cu2O+H2�� |
| ����d | ���£�N2H4����ԭ���Ƶ�Cu��OH��2 |
��C��s��+$\frac{1}{2}$O2��g���TCO��g������H=-110.5kJ•mol-1
��Cu��s��+$\frac{1}{2}$O2��g���TCuO��s������H=-157kJ•mol-1
��a�������Ȼ�ѧ����ʽ�ǣ�2CuO��s��+C��s��=Cu2O��s��+CO��g����H=+34.5kJ/mol��
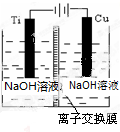
��2������c�������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ��
�����ӽ���ĤΪ�����ӽ���Ĥ������������������õ�ص�������ӦʽΪ2Cu-2e-+2OH-=Cu2O+H2O���Ѽ�������pHֵ�����������С�����䡱����
��3������dΪ������������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ4Cu��OH��2+N2H4$\frac{\underline{\;\;��\;\;}}{\;}$2Cu2O+N2��+6H2O��
��4������ͬ���ܱ������У������Ϸ����Ƶõ�����Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H��0��ˮ������Ũ����ʱ��t�仯���±���ʾ��
| ��� | 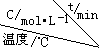 | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
��ʵ���ǰ20min��ƽ����Ӧ���� v��O2��=3.5��10-5mol/��L•min����
| ʵ�� ��� | ���� ����/g | ���� ״̬ | c��H2SO4�� /mol•L-1 | V��H2SO4��/mL | ��Һ�¶�/�� | ������ʧ��ʱ��/S | |
| ��Ӧǰ | ��Ӧ�� | ||||||
| 1 | 0.10 | ˿ | 0.5 | 50 | 20 | 34 | 500 |
| 2 | 0.10 | ��ĩ | 0.5 | 50 | 20 | 35 | 50 |
| 3 | 0.10 | ˿ | 0.7 | 50 | 20 | 36 | 250 |
| 4 | 0.10 | ˿ | 0.8 | 50 | 20 | 35 | 200 |
| 5 | 0.10 | ��ĩ | 0.8 | 50 | 20 | 36 | 25 |
| 6 | 0.10 | ˿ | 1.0 | 50 | 20 | 35 | 125 |
| 7 | 0.10 | ˿ | 1.0 | 50 | 35 | 50 | 50 |
| 8 | 0.10 | ˿ | 1.1 | 50 | 20 | 34 | 100 |
| 9 | 0.10 | ˿ | 1.1 | 50 | 20 | 44 | 40 |
��1��ʵ��4��5�������������Է�Ӧ������Ӱ�죻��������Խ��Ӧ����Խ�죬�ܱ�����һ������һ��ʵ����1��2����ʵ����ţ���
��2����ǰ4��ʵ���У��ܱ�����Ӧ��Ũ�ȶԷ�Ӧ���ʲ���Ӱ���ʵ����1��3��4������ţ���
��3����ʵ����Ӱ�췴Ӧ���ʵ��������ػ����¶ȣ���ʵ�������6��7��
��4��ʵ���У���Ӧǰ����Һ���¶ȱ仯ֵ��Լ15�棩������Ʋ���ԭ��Ӧ��������ͬ���ų���������ͬ��
��ij��ҵ��ˮ����Ҫ����Cr3+��ͬʱ������������Fe3+��Al3+��Ca2+��Mg2+�ȣ������Խ�ǿ��Ϊ�������ã�ͨ�������������̴�����
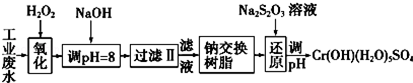
ע�����������ӳ�����������������ʽ��ȫ����ʱ��Һ��pH������
| �������� | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 | Al��OH��3 | Cr��OH��3 |
| pH | 3.7 | 9.6 | 11.1 | 8 | 9����9�ܽ⣩ |
A��Na2O2 B��HNO3 C��FeCl3 D��KMnO4
��2������NaOH��Һ������ҺpH=8ʱ����ȥ��������AB����������ѡ���֪�����ӽ�����֬��ԭ����Mn++nNaR-��MRn+nNa+���˲�������������ȥ������������CD����������ѡ�
A��Fe3+ B�� Al3+ C��Ca2+ D��Mg2+
��3����ԭ�����У�ÿ����0.8mol Cr2O72-ת��4.8mol e-���÷�Ӧ���ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�����������£����۸���Ҫ��Cr2O72-��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O72-�ķ�ˮ���÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr��OH��3������
��1�����ʱ�ܷ���Cu�缫������Fe�缫�����ܣ���ܡ����ܡ�����������������������Cu2+����ʹCr2O72-��ԭ���ͼ�̬��
��2�����ʱ����������Һ��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
��3�������£�Cr��OH��3���ܶȻ�Ksp=1��10-32����Һ��pHӦΪ5ʱ����ʹc��Cr3+������10-5 mol•L-1��
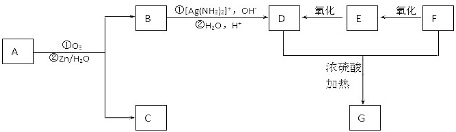
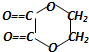 +2H2O����Ӧ������������Ӧ��D��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��HOOCCOOH+2NaHCO3=NaOOCCOONa+2CO2��+2H2O��
+2H2O����Ӧ������������Ӧ��D��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��HOOCCOOH+2NaHCO3=NaOOCCOONa+2CO2��+2H2O��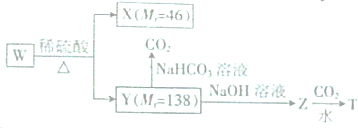
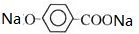 +CO2+H2O��
+CO2+H2O��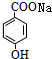 +NaHCO3��
+NaHCO3��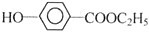
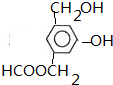 ��
��