��Ŀ����
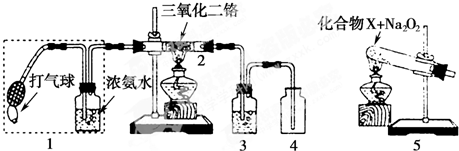
����ͼ������ijѧУ����ʦ�Ʊ�NH3����������ʵ��ʱ�ĸĽ�װ�ã���ͼ�װ�������װ�ã���ȡ2g �����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g �������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mL Ũ��ˮ�����ձ���ʢ���з�̪��Һ��ˮ����Ũ��ˮ�����Թ���������۲쵽�Թ��ڷ������ҷ�Ӧ���д������ݣ�
������������NH3��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�������Ԥ����2mLH2O����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3 ��ij�����ʣ���Ҫ��ش��������⣺
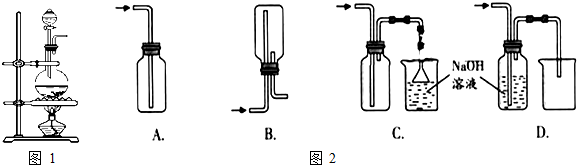
��1����ѧ��ѧ�̲�����������O2��ͬ���Ʊ�װ����������ȡNH3�ģ��û�ѧ����ʽΪ��
��2��������ijͬѧ��������ʦ����ͼ����ȡNH3��ԭ��������е�������
����NH3?H2O ����ƽ��NH3+H2O?NH3?H2O?NH
+ 4 |
����NH3?H2O ����ƽ��NH3+H2O?NH3?H2O?NH
+ 4 |
��Na0H����ˮʱ���ȣ�ʹ��ϵ���¶����ߣ�NH3���ܽ�ȼ�С
��NH4Cl��NaOH �ڴ�����¿ɷ�Ӧ����NH3����NH
+ 4 |
| ||
��NH4Cl ��ֽ��ͷų�NH3
��3��ͼ���е�NH4Cl ��NaOH ���������ܷ���CaO�������
��4������ж�ͼ������ƿ������NH3��
��5��ͼ���н�ͷ�ι��е�ˮ������ƿ�۲쵽��������
������ͼ2��ʾ����B����װ��500mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����ԭ���������NO2��NO�����ʵ���֮��Ϊ
ͨ��������C������0.5a mL������Թ�A�г���ͨ��������A�п��ܹ۲쵽�������ǣ�
���Թ�A�г�������ʱֹͣͨ��������Ȼ���Թ�ȡ��ˮ�ۣ�ˮ��B����Һ�����ʵ���Ũ��Ϊ
���㣺������ȡ������,����ʵ�����Ʒ�,���������Ʊ�ԭ����װ��ѡ��
ר�⣺ʵ����
����������1��ʵ�����ü�����κͼ�Ĺ���������ȡ����������װ������O2װ����ͬ��
��2���ڰ�ˮ�д���ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-������ƽ���ƶ���ԭ�����н��
��3����������ˮ��Ӧ�����������ƣ��ҷ�Ӧ�з��������������ڰ��������ɣ�
��4����ˮ�ʼ��ԣ���̪�����Ժ�ɫ��
��5��NH3�����ܽ���ˮ��1���ˮ�ܽ�700���������
������3NO2+2H2O=2HNO3+NO����������������С���������������С�����ò�������������������������������NO����������֮�ȵ������ʵ���֮�ȣ�
ʣ��NO������ͨ���������ᷢ����4NO+3O2+2H2O=4HNO3��ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
���ݵ�Ԫ���غ㣬��Ԫ���غ��֪n��HNO3��=n��NO2��+n��NO�����ٸ���c=
�������������Ũ�ȣ�
��2���ڰ�ˮ�д���ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-������ƽ���ƶ���ԭ�����н��
��3����������ˮ��Ӧ�����������ƣ��ҷ�Ӧ�з��������������ڰ��������ɣ�
��4����ˮ�ʼ��ԣ���̪�����Ժ�ɫ��
��5��NH3�����ܽ���ˮ��1���ˮ�ܽ�700���������
������3NO2+2H2O=2HNO3+NO����������������С���������������С�����ò�������������������������������NO����������֮�ȵ������ʵ���֮�ȣ�
ʣ��NO������ͨ���������ᷢ����4NO+3O2+2H2O=4HNO3��ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
���ݵ�Ԫ���غ㣬��Ԫ���غ��֪n��HNO3��=n��NO2��+n��NO�����ٸ���c=
| n |
| V |
���
�⣺����1��ʵ����������ع���Ͷ������̹�����ø�����ؼ�����O2������O2��ͬ���Ʊ�װ���ư�����ʵ�����ü����Ȼ�狀���ʯ�ҵĹ���������ȡ��������Ӧ����ʽΪ��2NH4Cl+Ca��OH��2
2NH3��+2H2O+CaCl2��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
2NH3��+2H2O+CaCl2��
��2�����ڰ�ˮ�д���ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�������������������������ӵ�Ũ�ȣ�ʹƽ�������ƶ����Ӷ�ʹNH3�ݳ����ʢ���ȷ��
�ڼ�NH4Cl��������Һ�а�������Ũ�ȣ�ʹƽ�������ƶ����ʢ���ȷ��
��NaOH����ˮ�ų�������ʹ��ϵ�¶����ߣ�ʹNH3���ܽ�ȼ�С���ݳ����ʢ���ȷ��
��NH4Cl��NaOH�ڴ�����¿ɷ�Ӧ����NH3�����ӷ���ʽΪ��NH4++OH-
NH3��+H2O���ʢ���ȷ��
�ݼ���NH4Cl�ֽ������Ȼ���Ͱ������Ȼ���Ͱ������ܹ���������Ȼ�泥�����ð������ʢݴ���
�ʴ�Ϊ���٢ڢۢܣ�
��3��CaO��ˮ��Ӧ�����������ƣ����������ܹ���������������ӣ��Ҹ÷�ӦΪ���ȷ�Ӧ�������˰������ܽ�ȣ������ڰ��������ɣ����Կ��������ƴ����������ƣ�
�ʴ�Ϊ���ܣ�
��4������ƿ�ڵİ����ռ���ʱ���������ݵ��ձ��У��������ձ��е�ˮ������ɰ�ˮ�Լ��ԣ�����ʹ��ɫ�ķ�̪��죬
�ʴ�Ϊ���ձ��ڵ���Һ��죻
��5����ͷ�ι��е�ˮ������ƿ������NH3��������ˮ����ƿ��ѹǿ��С����������
�ʴ�Ϊ��������� ��������ˮ��
��������NO2��NO�Ļ�����壨��״�����Թ�A��ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����
3NO2+2H2O=2HNO3+NO��������١�V
3 2
V��NO2�� amL-0.5amL=0.5amL
��ã�V��NO2��=
=0.75amL��
��V��NO��=amL-0.75amL=0.25mL��
��ԭ���������NO2��NO�����ʵ���֮��Ϊ��n��NO2����n��NO��=V��NO2����V��NO��=0.75amL��0.25mL=3��1��
ʣ������ΪNO������ͨ���������ᷢ��4NO+3O2+2H2O=4HNO3����ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
V��NO2��=0.75amL����Ԫ���غ��֪n��HNO3��=n��NO2��+n��NO��=
��10-3mol��
��c��HNO3��=
=
mol/L��
�ʴ�Ϊ��3��1����ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2�����ڰ�ˮ�д���ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�������������������������ӵ�Ũ�ȣ�ʹƽ�������ƶ����Ӷ�ʹNH3�ݳ����ʢ���ȷ��
�ڼ�NH4Cl��������Һ�а�������Ũ�ȣ�ʹƽ�������ƶ����ʢ���ȷ��
��NaOH����ˮ�ų�������ʹ��ϵ�¶����ߣ�ʹNH3���ܽ�ȼ�С���ݳ����ʢ���ȷ��
��NH4Cl��NaOH�ڴ�����¿ɷ�Ӧ����NH3�����ӷ���ʽΪ��NH4++OH-
| ||
�ݼ���NH4Cl�ֽ������Ȼ���Ͱ������Ȼ���Ͱ������ܹ���������Ȼ�泥�����ð������ʢݴ���
�ʴ�Ϊ���٢ڢۢܣ�
��3��CaO��ˮ��Ӧ�����������ƣ����������ܹ���������������ӣ��Ҹ÷�ӦΪ���ȷ�Ӧ�������˰������ܽ�ȣ������ڰ��������ɣ����Կ��������ƴ����������ƣ�
�ʴ�Ϊ���ܣ�
��4������ƿ�ڵİ����ռ���ʱ���������ݵ��ձ��У��������ձ��е�ˮ������ɰ�ˮ�Լ��ԣ�����ʹ��ɫ�ķ�̪��죬
�ʴ�Ϊ���ձ��ڵ���Һ��죻
��5����ͷ�ι��е�ˮ������ƿ������NH3��������ˮ����ƿ��ѹǿ��С����������
�ʴ�Ϊ��������� ��������ˮ��
��������NO2��NO�Ļ�����壨��״�����Թ�A��ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����
3NO2+2H2O=2HNO3+NO��������١�V
3 2
V��NO2�� amL-0.5amL=0.5amL
��ã�V��NO2��=
| 0.5amL��3 |
| 2 |
��V��NO��=amL-0.75amL=0.25mL��
��ԭ���������NO2��NO�����ʵ���֮��Ϊ��n��NO2����n��NO��=V��NO2����V��NO��=0.75amL��0.25mL=3��1��
ʣ������ΪNO������ͨ���������ᷢ��4NO+3O2+2H2O=4HNO3����ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
V��NO2��=0.75amL����Ԫ���غ��֪n��HNO3��=n��NO2��+n��NO��=
| a |
| 22.4 |
��c��HNO3��=
| ||
| 0.5L |
| a |
| 11200 |
�ʴ�Ϊ��3��1����ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
| a |
| 11200 |
���������⿼���˰����Ļ�ѧ���ʡ�������ʵ�����Ʒ���֪ʶ����Ŀ�Ѷ��еȣ������漰��֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ����������������ۺ�Ӧ����ѧ֪ʶ��������Ҫ��ѧ���������հ������Ʊ�ԭ�����Ʊ�װ�ü���ѧ���ʣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ͼ��ʾ�������̶�������������������ƶ���M��N���������о�������Ӧ��A��g��+3B��g��?2C��g����H=-192kJ?mol-1����M��N�У��ֱ�ͨ��x mol A��y mol B�Ļ�����壬��ʼM��N�ݻ���ͬ�������¶Ȳ��䣮����˵����ȷ���ǣ�������
��ͼ��ʾ�������̶�������������������ƶ���M��N���������о�������Ӧ��A��g��+3B��g��?2C��g����H=-192kJ?mol-1����M��N�У��ֱ�ͨ��x mol A��y mol B�Ļ�����壬��ʼM��N�ݻ���ͬ�������¶Ȳ��䣮����˵����ȷ���ǣ�������| A����ƽ��ʱA�������������е����������ȣ���xһ������y |
| B����x��y=1��2����ƽ��ʱ��M�е�ת���ʣ�A��B |
| C����x��y=1��3����M�зų�����172.8 kJʱ��A��ת����Ϊ90% |
| D����x=1.4��y=1��N�дﵽƽ��ʱ���Ϊ2L��CΪ0.4mol����Ӧ��ʼʱN�����Ϊ2.6L |
ȡ�����0.05mol?L-1��Ba��OH��2��Һ���ֱ�װ����Т٢ڢܱۢ�ŵ�4����ƿ�У����ټ�ˮϡ�͵�ԭ�����2�����ڢڢ��зֱ�ͨ��������CO2���������գ��ֱ��ڢ٢��еμӷ�̪��ָʾ����pH��ɫ��Χ��0-8-10-14�����ڢۢ��еμӼ�����ָʾ����PH��ɫ��Χ0-3.1-4.4-14������HCl��Һ�ֱ�ժ������4����Һ��������HCl��Һ������ǣ�������
| A���ڣ��٣���=�� |
| B����=��=��=�� |
| C���٣��ڣ��ۣ��� |
| D���ڣ��ۣ��٣��� |