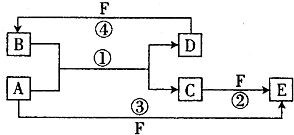
��Ŀ����
һ���¶��£���3molA�����1molB����ͨ��һ�ܱ������У��������·�Ӧ��3A��g��+B ��g��?xC��g��������������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L������д���пհף�
��1��x= �� 1min�ڣ�B��ƽ����Ӧ����Ϊ ��
��2������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڡ��������ڡ���С�ڡ�����
��3��ƽ�������У�C���������Ϊ22%����ά������ѹǿ���䣬�ﵽƽ��ʱC��������� 22%��������ڡ��������ڡ���С�ڡ�����
��4�����ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ���n��A����n ��B����n��C��֮��Ӧ����Ĺ�ϵʽ ��
��1��x=
��2������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��
��3��ƽ�������У�C���������Ϊ22%����ά������ѹǿ���䣬�ﵽƽ��ʱC���������
��4�����ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ���n��A����n ��B����n��C��֮��Ӧ����Ĺ�ϵʽ
���㣺��ѧƽ���Ӱ������,��Чƽ��
ר�⣺��ѧƽ��ר��
��������1����������ʽ������ 3A��g��+B��g���TxC��g��
��ʼ��mol/L����1.5 0.5 0
ת����mol/L����0.6 0.2 0.2x
ƽ�⣨mol/L����0.9 0.3 0.4 0.2x=0.4��x=2���Դ˽����⣮
��2�����ŷ�Ӧ�Ľ��У���Ӧ������С�����ݷ�Ӧ��ʵ�����ش�
��3����ά������ѹǿ���䣬Ӧ��С�����ƽ�����������ƶ���
��4�����º����£��ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ�����ת������ʼ����ͬ��
��ʼ��mol/L����1.5 0.5 0
ת����mol/L����0.6 0.2 0.2x
ƽ�⣨mol/L����0.9 0.3 0.4 0.2x=0.4��x=2���Դ˽����⣮
��2�����ŷ�Ӧ�Ľ��У���Ӧ������С�����ݷ�Ӧ��ʵ�����ش�
��3����ά������ѹǿ���䣬Ӧ��С�����ƽ�����������ƶ���
��4�����º����£��ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ�����ת������ʼ����ͬ��
���
�⣺��1������������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����
A�ķ�Ӧ����Ϊ
=0.6mol/��L?min������ͬ���ʱ�ʾ�Ļ�ѧ��Ӧ����֮�ȵ���ϵ��֮�ȣ�����B�ķ�Ӧ����=0.2mol/��L?min����
�ɷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪��B��ƽ����Ӧ����Ϊ0.2mol/��L?min����x=2��
�ʴ�Ϊ��2��0.2mol/��L?min����
��2�����ŷ�Ӧ�Ľ��У���Ӧ������С������Ӧ��2min�ﵽƽ�⣬��Ӧ����ӦС��1minʱ��1minʱ��C��Ũ��Ϊ0.4mol/L����ƽ��ʱC��Ũ��С��0.8mol/L��
�ʴ�Ϊ��С�ڣ�
��3����������̶�ʱC���������Ϊ22%����ά������ѹǿ���䣬Ӧ��С�����ƽ�����������ƶ�����ﵽƽ��ʱC�������������22%��
�ʴ�Ϊ�����ڣ�
��4�����º����£��ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ�����ת������ʼ����ͬ����nA+
nC=3��nB+
nC=1��
�ʴ�Ϊ��nA+
nC=3��nB+
nC=1��
A�ķ�Ӧ����Ϊ
| ||
| 1min |
�ɷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪��B��ƽ����Ӧ����Ϊ0.2mol/��L?min����x=2��
�ʴ�Ϊ��2��0.2mol/��L?min����
��2�����ŷ�Ӧ�Ľ��У���Ӧ������С������Ӧ��2min�ﵽƽ�⣬��Ӧ����ӦС��1minʱ��1minʱ��C��Ũ��Ϊ0.4mol/L����ƽ��ʱC��Ũ��С��0.8mol/L��
�ʴ�Ϊ��С�ڣ�
��3����������̶�ʱC���������Ϊ22%����ά������ѹǿ���䣬Ӧ��С�����ƽ�����������ƶ�����ﵽƽ��ʱC�������������22%��
�ʴ�Ϊ�����ڣ�
��4�����º����£��ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ�����ת������ʼ����ͬ����nA+
| 3 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��nA+
| 3 |
| 2 |
| 1 |
| 2 |
�����������ۺϿ��黯ѧƽ��ļ��㣬��Ŀ�ѶȲ���ע����������ʽ�������⣬�״���Ϊ��4����ע���Чƽ��������Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
���б��ﲻ��ȷ���ǣ�������
| A��NaHS��ˮ�еĵ��뷽��ʽΪ��NaHS�TNa++HS-��HS-�TH++S2- |
| B��ͬ���ʵ���Ũ�ȵİ�ˮ�����ᷴӦ������ʱ���������V��NH3?H2O����V��HCl�� |
| C��Na2SO3��Һ�У�c��H+��+c��HSO3-��+2c��H2SO3��=c��OH-�� |
| D��ͬŨ�ȵ�������Һ�У�c��CH3COO-���Ĵ�С��CH3COONa��CH3COONH4��CH3COOH |
��֪25��ʱ�й�����ĵ���ƽ�ⳣ��������������й�˵����ȷ���ǣ�������
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�� ������25�棩 |
1.8��l0-5 | 4.9��l0-10 | K1=4.3��l0-7 K2=5.6��l0-11 |
| A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪpH��NaCN����pH��Na2CO3����pH��CH3COONa�� |
| B��amol?L-1HCN��Һ��amol?L-1NaOH��Һ�������Ϻ�������Һ�Լ��ԣ�pH��7������c ��OH-����c��H+����c��Na+����c��CN-�� |
| C������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С |
| D��NaHCO3��Na2CO3�����Һ�У�һ����c��Na+��+c��H+��=c��OH-��+c��HCO3-��+c��CO32-�� |
X��Y��ԭ����������4����������Ԫ�أ����ǵ�����Xm+��Yn-������ͬ�ĺ�������Ų����й�X��Y����������ȷ���ǣ�������
| A��ԭ��������С��X��Y |
| B��Xm+��Yn-���Ӱ뾶��С��Yn-��Xm+ |
| C�������ڱ���X��Y�ڲ�ͬ���� |
| D�������ڱ���X��Y��������֮��Ϊ��8-��m+n�� |
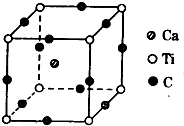 ������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ�
������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ� W��X��Y��Z��RΪǰ������Ԫ����ԭ��������������W��Y�γɳ����³�Һ̬�Ļ�����G��X����spm��m=1��2��3���ӻ���ʽ��X�γ�һϵ��X�Ļ����X�ĵ����ڸ�����������G��Ӧ����W���ʺ���̬�����W�ĵ�����Z�ĵ����ڵ�ȼ�����ʱ���ܷ�Ӧ����1��1��ˮ��ҺΪ�������Rԭ�ӵļ۵��Ӳ��δ�ɶԵ�����Ϊ4���ش��������⣺
W��X��Y��Z��RΪǰ������Ԫ����ԭ��������������W��Y�γɳ����³�Һ̬�Ļ�����G��X����spm��m=1��2��3���ӻ���ʽ��X�γ�һϵ��X�Ļ����X�ĵ����ڸ�����������G��Ӧ����W���ʺ���̬�����W�ĵ�����Z�ĵ����ڵ�ȼ�����ʱ���ܷ�Ӧ����1��1��ˮ��ҺΪ�������Rԭ�ӵļ۵��Ӳ��δ�ɶԵ�����Ϊ4���ش��������⣺