��Ŀ����
4������ˮ�������ȵIJⶨ���������ʵ�飺ȡһ��������ˮ������ƿ�У�����������KI��Һ��2��3��ָʾ������0.0010mol•L-1��Na2S2O3��Һ�ζ���Ӧ�����ɵ�I2��I2����ԭΪI-��S2O32-������ΪS4O62-�����������ĵ�Na2S2O3��Һ��������������ˮ�������ȵ�Ũ�ȣ���ش��������⣺��1��д��Na2S2O3��I2��Ӧ�����ӷ���ʽI2+2S2O32-=2I-+S4O62-��
��2���ζ���ʹ�õ�ָʾ���ǵ�����Һ���ζ��յ����������ɫ��Һǡ�ñ�Ϊ��ɫ�Ұ�����ڲ��ָ���
��3����С��ͬѧ��ʵ���������±���
| ʵ����� | ����ˮ����� | KI��Һ����� | ���ĵ�Na2S2O3��Һ����� |
| 1 | 10.00mL | 10.00mL | 19.96mL |
| 2 | 10.00mL | 10.00mL | 20.04mL |
��4����ʵ���е���ƿ������ˮϴ����δ������ˮϴ�ӣ���ʵ��ֵ���ڣ�����ڡ�С�ڻ���ڡ���ʵ��ֵ��
���� ��1��Na2S2O3��I2��Ӧ��I2����ԭΪI-��S2O32-������ΪS4O62-��д�����ӷ���ʽ��I2+2S2O32-=2I-+S4O62-��
��2���ζ���ʹ�õ�ָʾ���ǵ�����Һ�����ⷴӦ��ȫʱ����Һ����ɫǡ�ñ�Ϊ��ɫ�Ұ�����ڲ��ָ���
��3���������ӷ���ʽ��I2+2S2O32-=2I-+S4O62-��Cl2+2I-=I2+2Cl-��֪��ϵʽ��Cl2��I2��2S2O32-����n��Cl2�������c��Cl2����
��4��ʵ���е���ƿ������ˮϴ����δ������ˮϴ�ӣ�����ʹ����ˮ�������ӣ���õ�ʵ��ֵ����ʵ��ֵ��
��� �⣺��1��Na2S2O3��I2��Ӧ��I2����ԭΪI-��S2O32-������ΪS4O62-��д�����ӷ���ʽ��I2+2S2O32-=2I-+S4O62-��
�ʴ�Ϊ��I2+2S2O32-=2I-+S4O62-��
��2���ζ���ʹ�õ�ָʾ���ǵ�����Һ�����ⷴӦ��ȫʱ����Һ����ɫǡ�ñ�Ϊ��ɫ�Ұ�����ڲ��ָ����ﵽ�յ㣬
�ʴ�Ϊ��������Һ����ɫ��Һǡ�ñ�Ϊ��ɫ�Ұ�����ڲ��ָ���
��3���������ӷ���ʽ��I2+2S2O32-=2I-+S4O62-��Cl2+2I-=I2+2Cl-��֪��ϵʽ��Cl2��I2��2S2O32-��
���ĵ�Na2S2O3��Һ�����Ϊ����19.96mL+20.04mL����2=20.00mL��v��Na2S2O3��=20.00 mL��c��Na2S2O3��=0.0010mol•L-1��
��n��S2O32-��=c��Na2S2O3����v��Na2S2O3��=0.0010mol•L-1��20.00��10-3 L=2��10-5 mol��
����Cl2��I2��2S2O32-��n��Cl2��=1��10-5 mol��
c��Cl2��=$\frac{n��C{l}_{2}��}{V������ˮ��}$=1��10-5 mol�£�10.00��10-3 L��=0.0010 mol•L-1��
�ʴ�Ϊ��0.0010 mol•L-1��
��4��ʵ���е���ƿ������ˮϴ����δ������ˮϴ�ӣ�����ʹ����ˮ�������ӣ���õ�ʵ��ֵ����ʵ��ֵ��
�ʴ�Ϊ�����ڣ�
���� ���⿼����������ԭ�ζ���ָʾ����ʹ�á��ζ��յ���жϣ��ζ�����ʱҪע���ù�ϵʽ�����Ŀ�Ѷ����У�

| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO42- | HCO3- |
| ����/mg•L-1 | 9360 | 83 | 160 | 1100 | 16000 | 1200 | 118 |
��2�������Ǻ�ˮ���õ���������õ�ˮ��ԭ��ͼ���缫Ϊ���Ե缫��������������⣺
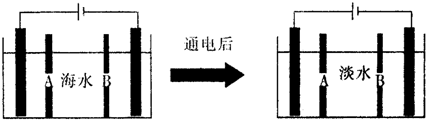
�������ӽ���Ĥ��ָB����A��B����
��д��ͨ����������ĵ缫��Ӧʽ��2Cl--2e-=Cl2�������һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽCa2++OH-+HCO3-=CaCO3��+H2O��
��3��ˮ�ľ�����������������ˮ�ľ������û��������������ȣ���ˮ�������������������ˮ�������dz�ȥˮ�еĸ����Ӻ�þ���ӣ����ӽ�����������ˮ�ij��÷������۱�ϩ������һ�����ӽ�����֬��д���۱�ϩ���Ƶ���Ľṹ��ʽCH2=CHCOONa��
��4�����á���������������Ũ��ˮ�д���Br2�����ô������գ������������Ҫ��Ӧ�ǣ�Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������80g Br2ʱת�Ƶĵ���Ϊ$\frac{5}{6}$mol��
�ٵ��� ����ά�� ����֬ ������ �۾���ϩ��
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �٢ڢ� | D�� | ȫ�� |
| A�� | �߷��ӷ���Ĥ | B�� | �����۾� | C�� | ����˿ | D�� | ������� |

| A�� | ��÷����廯�������ʽ��ͬ�����������༰��Ŀ����ͬ�ұ����ϵ�һ�ȴ���ֻ�����ֵ��л���Ľṹ��4�֣������ǿռ��칹�� | |
| B�� | ������ֻ�ܼӾ۳ɸ߷��ӻ����� | |
| C�� | ������������5mol���������ӳɷ�Ӧ | |
| D�� | 1mol��������������Na2CO3��Һ��Ӧ��������3molCO32- |
| A�� | ���ױ� | B�� | ���� | C�� | һ�ȶ�ϩ | D�� | ���ȱ��� |
| A�� | C20H30O2��C22H30O2 | B�� | C22H32O2��C20H30O2 | ||
| C�� | C22H30O2��C20H30O2 | D�� | C20H30O2��C22H32O2 |

 ��
��