��Ŀ����
2��CuCl2��Һ�к���������FeCl2��Ϊ�Ʊ�����CuCl2•2H2O���ⶨʵ�鲽�����£�
������������⣺
��1�������˵���������C��
A��K2Cr2O7 B��NaClO C��H2O2D��KMnO4
��2������Y��CuCO3��CuO��Cu��OH��2��
��3������Y���Ӷ���ȥFe3+���йػ�ѧ����ʽ��FeCl3+3H2O?Fe��OH��3+3HCl��CuCO3+2HCl�TCuCl2+H2O+CO2����
��4��Ҫ��CuCl2•2H2O�Ƶ���ˮCuCl2��Ӧ���Ƶ�һ��������_��HCl�������м��ȣ�
���� CuCl2��Һ�к�����������FeCl2������������Ӧ���������������ӣ�Ϊ��������������ͭ����Ϊ�����ӣ���������ͭ��̼��ͭ���ʽ̼��ͭ������ҺPHʹ������ȫ�����������˵õ��Ȼ�ͭ��Һ������Ũ������ȴ�ᾧ����ϴ�ӵõ�������CuCl2•2H2O���壬
��1�������������ӵ����������������µ����ʣ�
��2��Y���ڵ���pH��ע�ⲻ���������ʣ�
��3��pH����������������ˮ��������������������
��4���Ȼ�ͭ��ˮ�⣬����ʹӦ����ͭ���ӵ�ˮ�⣮
��� �⣺CuCl2��Һ�к�����������FeCl2������������Ӧ���������������ӣ�Ϊ��������������ͭ����Ϊ�����ӣ���������ͭ��̼��ͭ���ʽ̼��ͭ������ҺPHʹ������ȫ�����������˵õ��Ȼ�ͭ��Һ������Ũ������ȴ�ᾧ����ϴ�ӵõ�������CuCl2•2H2O���壬
��1�������������ӵ����������������µ����ʣ�
A��K2Cr2O7���������������ӵ���������ӡ����������ʣ���A����
B��NaClO������ͭ���ӣ������������ӣ���B����
C��H2O2�����������ӣ��������ⱻ����ԭΪˮ���������µ����ʣ���C��ȷ��
D��KMnO4������ͭ���ӣ�����������ӡ������ӵȣ���D����
�ʴ�Ϊ��C��
��2�����ٹ������ᴿ�Ȼ�ͭ������Y��Ŀ����Ϊ�˵�����Һ������Դ�ʹFe3+������ȫ�������������µ����ʷ������������ƻ������������������ӣ�ͭ���ܵ�����ҺPH��Cu2��OH��2CO3���Ժ�����Һˮ���������Һ��Ӧ������ҺPH�������µ����ʣ�
�ʴ�Ϊ��CuCO3��CuO��Cu��OH��2��
��3��pH����������������ˮ����������������������Ӧ�ķ���ʽΪFeCl3+3H2O?Fe��OH��3+3HCl�������CuCO3���ɷ���CuCO3+2HCl�TCuCl2+H2O+CO2����
�ʴ�Ϊ��FeCl3+3H2O?Fe��OH��3+3HCl��CuCO3+2HCl�TCuCl2+H2O+CO2����
��4���Ȼ�ͭ��ˮ�⣬����ʹӦ����ͭ���ӵ�ˮ�⣬��ֹ����������ͭ��������������HCl�������м��ȣ��ʴ�Ϊ����HCl�������м��ȣ�
���� ���⿼�����ʵ��Ʊ���Ϊ��Ƶ���㣬��Ŀ��ʵ��ķ�������ѧ�����������������֪ʶ��ע�����ʵķ��롢�ᴿ�ͻ�ѧʵ�����������֪ʶ��ע�����ʵ���ԭ���Լ������������Ѷ��еȣ�

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�| A�� | 0.3 mol H2SO4 | B�� | 1 mol H2O | C�� | 0.5 mol �� | D�� | 3 mol ��ԭ�� |
H2��g��+Br2��g��?2HBr��g����֪����1molH2��2molBr2ʱ���ﵽƽ�������a molHBr�������±�����֪���������ͬ�������£��ı���ʼ�����������д���пհף�
| ��� | ��ʼ״̬ | ƽ��ʱHBr���ʵ�����mol�� | ||
| H2 | Br2 | HBr | ||
| ��֪ | 1 | 2 | 0 | a |
| ��1�� | 2 | 4 | 0 | 2a |
| ��2�� | 0 | 0.5 | 1 | 0.5a |

 ��E�����������ŵ�����Ϊȩ�����Ȼ���
��E�����������ŵ�����Ϊȩ�����Ȼ���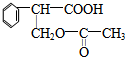 +H2O��
+H2O�� ��
�� ����д��һ�ּ��ɣ�
����д��һ�ּ��ɣ� ���������Ǽ��ֳ����㾫����Ҫ�ɷ֣�
���������Ǽ��ֳ����㾫����Ҫ�ɷ֣� ��
�� ��
��

 CO+3H2�� ��ԭ�����Ʊ������л��е�CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ����ӦCO��g��+H2O��g��?CO2��g��+H2��g����ʵ�֣���֪1100Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.64����ҪʹCO��ת���ʳ���80%������ʼ����c��H2O����c��CO��������5.8��1��
CO+3H2�� ��ԭ�����Ʊ������л��е�CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ����ӦCO��g��+H2O��g��?CO2��g��+H2��g����ʵ�֣���֪1100Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.64����ҪʹCO��ת���ʳ���80%������ʼ����c��H2O����c��CO��������5.8��1�� 6xH2O+��2x+3��N2��
6xH2O+��2x+3��N2��


